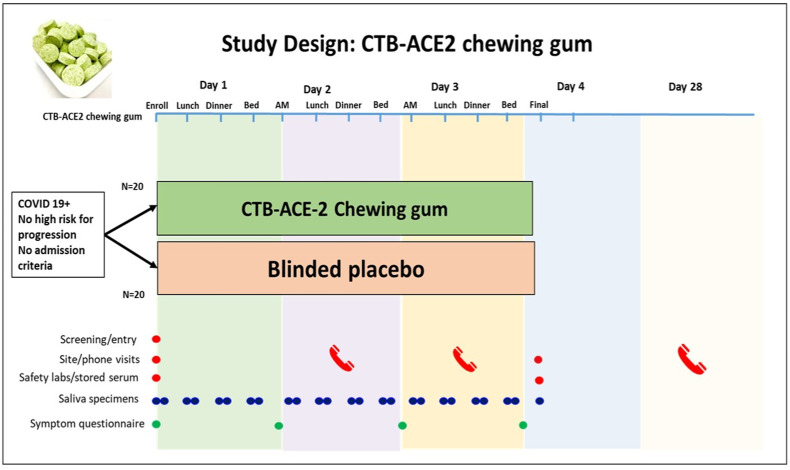
Fig. 8.
CTB-ACE2 chewing gum Phase I/II clinical study design. Based on FDA approval of IND (154897) Phase I/II placebo-controlled, double-blind randomized CTB-ACE2 or placebo gum study is in progress. Study duration is four days with 13 gums total, four gums each in days 1–3. An unstimulated whole saliva sample will be collected before eating or drinking or brushing teeth. Subjects will chew the CTB-ACE2 chewing gum/placebo gum (study product containing 2 g of CTB-ACE2 or placebo) for 10 min and then immediately provide a post-treatment 2–5 mL saliva sample in pre-labeled salivary collection tubes. Viral load will be quantified by qPCR or protein (N or spike) quantitation.