Abstract
Cardiovascular disease is the primary cause of death around the world. For almost two decades, cell therapy has been proposed as a solution for heart disease. In this article, we report on the “state‐of‐play” of cellular therapies for cardiac repair and regeneration. We outline the progression of new ideas from the preclinical literature to ongoing clinical trials. Recent data supporting the mechanics and mechanisms of myogenic and paracrine therapies are evaluated in the context of long‐term cardiac engraftment. This discussion informs on promising new approaches to indicate future avenues for the field.
Keywords: cardiac, cellular therapy, mesenchymal stem cells, pluripotent stem cells, stem cells
Cardiovascular disease is the primary cause of death around the world. For almost two decades, cell therapy has been proposed as a solution for heart disease. In this article, we report on the “state‐of‐play” of cellular therapies for cardiac repair and regeneration.
Significance statement.
For almost two decades, cell therapy has been promised as a solution for cardiac disease. The present study summarizes recent progress in the field of paracrine and stem cell‐derived cardiomyocytes for cardiac repair and regeneration. The authors explore how the field is evolving and provide context for future advances in stem cell mediated therapeutics.
1. INTRODUCTION
For over two decades, cell therapy has been promised as a solution for heart disease. The rationale for this focus is obvious. Cardiovascular disease is the primary cause of death around the world. In 2019, cardiac disease accounted for 32% of all deaths. 1 The World Health Organization believes this toll will rise from 17 million deaths per year to over 23 million by 2030. Therapies for cardiovascular disease are also becoming more expensive. Current forecasts suggest the total cost for cardiac disease will rise from US$863 billion in 2010 to over US$1 trillion in 2030. 2 As such, any solution that can reverse this epidemic is welcome. The hope that a patient's own cells could be used to repair or replace injured tissue naturally captured the imagination of the public and researchers alike when it was first proposed.
Early reports helped to fan this fervor. Several preclinical studies in high impact journals showed that intramyocardial cell injection could improve heart function after myocardial infarction. Enthusiastic editorials embraced these results and extended the reports into the popular press. In retrospect, this hype led to unrealistic expectations. Badly designed and underpowered clinical trials followed. 3 , 4 Consequently, the last decade has been littered with several unsuccessful clinical trials and a growing skepticism that the field will ever deliver. Editorials have questioned the use of public funds in stem cell therapy for heart failure 5 and the European Society of Cardiology Working Group recently concluded the promise of cardiovascular cell therapy has not yet been fulfilled. 6
Although much of this criticism has been well earned, “unfulfilled” is not equivalent to “unfeasible” or “unrealistic” and several promising solutions have emerged over the past 20 years to provide reasons for optimism. This report will critically focus on this recent data to cover the “state‐of‐play” of cellular therapies for cardiac repair and regeneration. We will start by exploring the progression of promising new ideas from the preclinical literature to ongoing clinical trials. The evidence supporting paracrine based therapies will be evaluated in the context of long‐term cardiac engraftment. This discussion will inform upon the ability of transplanted cells to stimulate the growth of new myocardium. Finally, the growing challenge for cell delivery will be used to explore myocyte replacement strategies to indicate future avenues for the field.
1.1. Cell therapy for heart disease
The roots of cell therapy for heart disease date back to the 1908 Congress of Hematology Society in Berlin. At this meeting, the Russian histologist Alexander Maksimov proposed that a population of pluripotent bone marrow cells existed to renew and replenish blood cells. 7 Till and McCulloch later showed that these self‐renewing cells could be isolated from bone marrow 8 and opened the doors for bone marrow transplantation to treat genetic diseases 9 , 10 and hematological malignancies. 11 , 12 , 13 By the mid‐1990s, bone marrow transplant had become the standard of care. Cardiovascular researchers began to collaborate with hematologists to see if bone marrow cells could repair myocardium. In early 2001, several independent reports indicated that adult bone marrow cells could repair myocardial infarcts in mice 14 , 15 and touched off a tremendous interest in cardiac cell therapy. Since then, thousands of animals around the world have been injected with almost every cell type that could be isolated, grown, manipulated, or engineered in the lab.
To appreciate the scope of this progress, we reviewed the Medline literature from January 1, 2000 to 2021 (see Supplementary methods for more details). Our search focused on preclinical studies that administered a cell product to an animal model of ischemic injury as data generated from an in vivo model represents a clear indication that a technology is on the way toward development. As shown in Figure 1A, the number of publications progressively increased from 2000 to 2009 as more groups became engaged in the field and new technologies emerged. In recent years, straightforward injection of cells into animal models has declined which may a reflect growing interest in cell derived products (such as extracellular vesicles) or defined factors.
FIGURE 1.
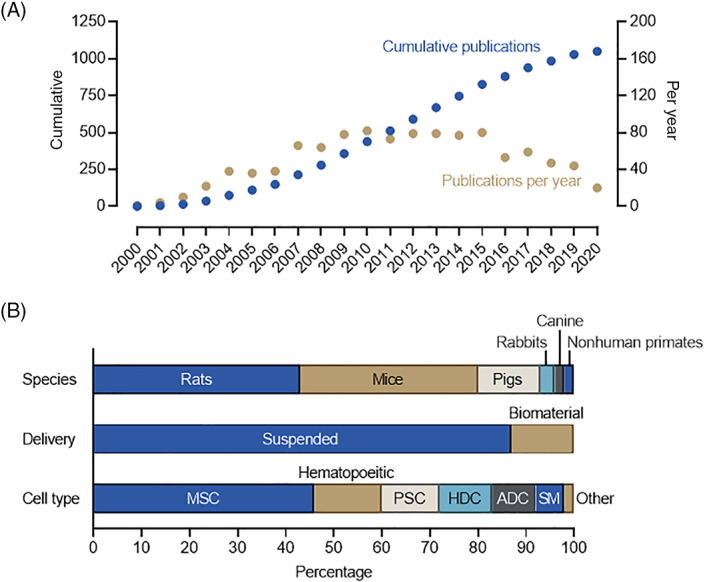
Analysis of the preclinical literature administering cell therapy to animal models of cardiac injury. A, The cumulative number and number per year of preclinical papers that administered cell therapy for heart failure. B, Breakdown of study proportion in terms of species used, delivery method and cell type administered. ADC, adipose derived cells; HDC, heart derived cells; MSC, mesenchymal stromal cells; PSC, pluripotent stem cells; SM, skeletal myoblasts
Controversy has also plagued the field particularly in studies using heart‐derived cells. Given early reports suggesting that very few transplanted bone marrow cells engraft and differentiate into working myocardium, it seemed logical that the benefits seen after cell injection might result from stimulation of endogenous heart stem cells. As such, several labs began to focus on isolating these endogenous heart stem cells. Using a candidate antigen approach, cardiac c‐Kit+ cells were suggested to be capable of clonogenic self‐renewal and differentiation into cardiac lineages. 16 This observation led to a substantial number of high profile papers supporting the view that resident cardiac c‐Kit+ cells regenerate injured myocardium and that ex vivo expanded heart c‐Kit+ cells could repair injured hearts. 17 But starting in 2009, reports began to emerge that suggested c‐Kit+ cells may not have a true cardiomyogenic ability. 18 , 19 , 20 The definitive study then came in 2014 where van Berlo and colleagues used Cre recombinase models to label c‐Kit+ cells and showed that less than 0.03% of all myocytes originated from c‐Kit+ cells during normal development. 21 They also showed that resident c‐Kit+ cells played little to no role in response to injury as only 0.016% or 0.007% of cardiomyocytes originated from resident c‐Kit+ cells after left coronary artery ligation or isoproterenol (a profibrotic stimulus), respectively. Confirmatory reports using multiple complimentary reporter genes have since shown that the contribution of resident heart c‐Kit+ cells to life‐long myocyte turnover or in situ regenerative responses to cardiac injury is functionally insignificant. 22 , 23 , 24 Many have since concluded that adult c‐Kit+ cells play no role in cardiomyogenesis. 25 Although antigenically selected and expanded c‐Kit+ cells have been shown to promote myocardial recovery, head‐to‐head comparisons with other cell products suggest this effect is modest. 26 , 27
In parallel to the troubled c‐Kit+ experience, a substantial body of evidence emerged supporting the use of a mixed heart‐derived cell population referred to as cardiosphere‐derived cells or explant‐derived cells. 28 , 29 This technology was initially developed by the Messina group to culture a mixed cell population from minced cardiac tissue. 30 These CD105+/CD45− cells of intrinsic cardiac origin have been shown to adopt a cardiomyocyte identity after transplant but the mechanism of benefit is largely through paracrine stimulation of endogenous repair by secreted cytokines and extracellular vesicles. 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 In contrast to the limited number of groups that published using c‐Kit+ cells, explant‐derived cells and their expanded progeny, cardiosphere‐derived cells, have been used by multiple (50+) independent research groups around the world. 29
As shown in Figure 1B, many of these preclinical proof‐of‐concept studies were performed in high throughput, low‐cost, small animal models (~81% mouse and rat models) with very few progressing to late‐stage nonhuman primate models (1%). Although most studies simply injected suspended cells into the animal model, biomaterials have been used in an increasing number of studies (13%) as a growing recognition emerged that all cell products struggle with long‐term engraftment. 38 Mesenchymal stromal cells (MSCs) were the most frequent cell type administered (46%) followed by hematopoietic cells (14%), pluripotent stem cells (PSC, 12%), heart derived cells (HDCs, 11%) and adipose‐derived cells (ADCs, 9%). Skeletal myoblasts enjoyed much early attention as this hardy noncardiac progenitor seemed ideally suited to engraft in the damaged heart. But concerns regarding life‐threatening heart rhythms sidelined this cell type. 39
This intense preclinical work translated into several clinical trials. As shown in Figure 2, a survey of clinical studies lists 82 registered trials administering “stem cells“ for “heart failure” (see Supplementary methods for more details). Trial registries started within 2 to 3 years of the initial reports exploring the effects of intramyocardial injected cells in animal models which speaks to the enthusiasm for this new technology (Figure 2A). Consistent with the preclinical literature, most of the trials involved administration of hematopoietic or MSCs (~70%; Figure 2B). Cell product choice also influenced when clinical trials were first registered (Figure 2C). Hematopoietic and skeletal muscle cell products were the first cell products investigated followed shortly by MSCs, HDCs, ADCs and, more recently, PSCs. Most studies (37%) were listed as “Completed” but there was a significant number listed as “Unknown” (17%), “Terminated” (11%), or “Withdrawn” (5%; Figure 2D). Only one study was listed as suspended (NCT03180450) with no explanation provided. In keeping with the time course of study registration, most completed studies were performed using a hematological cell product while PSCs had the greatest number of studies still listed as recruiting. Finally, phase 2 represented the greatest number of trials (45%) with phase 3 in the minority (7%).
FIGURE 2.
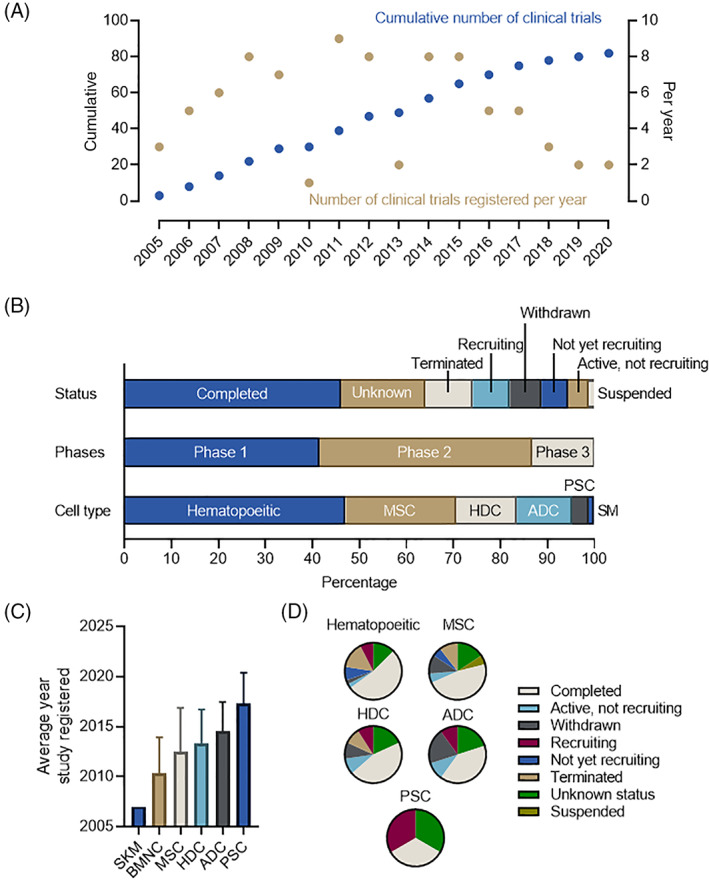
Analysis of the clinical trials listings administering cell therapy to heart failure patients. A, The cumulative number and number per year of clinical papers that administered cell therapy for heart failure. B, Breakdown of study proportion in terms of status (as of May 1, 2021), study phases, and cell type administered. C, Relationship between cell type administered and date the study first registered on ClinicalTrials.gov. Data is displayed as mean ± SD. D, Relationship between cell type administered and the clinical trial status. ADC, adipose derived cells; BMNC, bone marrow mononuclear cells; HDC, heart derived cells; MSC, mesenchymal stromal cells; PSC, pluripotent stem cells; SM, skeletal myoblasts
The conclusions from this experience are encouraging but have not yet provided a clear signal for benefit. Administration of cell products is safe with the clinical literature broadly confirming that intracoronary or intramyocardial injection of cells is safe and well tolerated. As outlined above, skeletal myoblasts provided the only clinical signal indicative of adverse events associated with cell administration (ie, cardiac arrhythmias) which, in retrospect, might have been detected in preclinical testing. 40 The risks of cell manufacturing should also not be discounted as rare events can occur (ie, tamponade associated with cardiac biopsy for heart c‐Kit+ cell isolation). 41 These insights have led to the development of the innovative cell types, clinical trial designs and delivery strategies which underlie recent attempts to develop new therapies for cardiovascular diseases.
1.2. The challenges of cardiac cell therapy
More than 95% of new drugs fail during clinical development. 42 These failures are often ascribed to the drug itself operating through a novel mechanism of action or an incomplete understanding of the disease pathogenesis. 42 , 43 , 44 Any early cell therapy for cardiac disease was inherently “first in class” but more importantly the understanding of how these cells might work was fundamentally flawed.
It was initially presumed that any injected cell would naturally adhere and migrate to areas of damage. We now know engraftment of any cell type is very modest at best with long‐term retention lucky to exceed 2% of the initial injectate. 45 , 46 , 47 This reality reflects: (a) adverse effects during administration, (b) clearance by blood vessels or lymphatics from an inherently vascular organ, (c) off target injection into the blood stream or cavity, (d) mechanical extrusion from the site of injection, and (e) the harsh hypoxic microenvironment where transplanted cells are expected to survive. Radiolabeling experiments have shown that only ~17% of cells are retained 1 hour after administration into the ideal milieu (ie, intramyocardial injection into a non‐perfused coronary ligation model). 48 Realistic models that mimic post intervention reperfused infarcts (ie, ischemic reperfusion injury) are even worse with only 12% persisting 1 hour after intramyocardial injection.
Our understanding of the mechanism by which transplanted cells promote myocardial repair has also become more refined. It is now known that improvements in cardiac function seen after any cell treatment come from immunomodulatory (macrophage polarization) and trophic (angiogenic, anti‐apoptotic, mitotic, and anti‐fibrotic) effects on resident tissue and not differentiation into new working myocardium. 28 , 38 This mechanism remains true even of pluripotent stem cell sources where the purported mechanism of action lies in direct replacement of lost myocytes with new pluripotent sourced cardiomyocytes. Several studies have since shown that the extracellular vesicles secreted by pluripotent stem cells and their daughter cardiomyocytes recapitulate a large portion of the therapeutic effects seen with their parent cells. 49 , 50 , 51 , 52 Similar to other cell types, long‐term engraftment of PSCs is limited yet the salutary effects of cell treatment effects persist. What remains uncertain is the importance of cell engraftment amid a backdrop of paracrine stimulated endogenous repair. Central to this controversy lies the ability of adult hearts to generate new myocardium.
1.3. Can adult myocytes reenter the cell cycle?
Unlike zebrafish and reptiles, mammalian cardiomyocytes exit the cell cycle soon after birth and lose the ability to generate new myocytes. This loss translates into an inability to compensate for the billions of myocytes lost after injury and contributes to cardiovascular mortality. Evolutionarily, this strategy makes sense as potentially catastrophic cardiac tumors are exceedingly rare (0.1% in autopsy series). 53 Recent work has shown that low level proliferation continues after birth and all myocytes are not locked into a static fate. The most compelling evidence comes from the radioactive carbon‐14 dating studies that showed ~40% of human cardiomyocytes renew during a normal lifespan. 54 This provides an annual renewal rate of 1% during the second decade of life that declines to 0.3% as individuals approach the age of 75 years. The source of these new myocytes are resident myocytes. 55 , 56 and not endogenous progenitor cells. Injury can increase myocyte reentry into the cell cycle 56 but recent work has shown this increase leads mainly to polyploidy (ie, a normally diploid cell acquires one or more additional sets of chromosomes) and not a substantial increase in new cardiomyocytes. 57
How and why these myocytes reenter the cell cycle is also becoming increasingly clear. Recent work has established that overexpression of small single‐stranded noncoding microRNAs (such as miR‐294 58 or miR302‐367 59 ) can increase cardiomyocyte proliferation. This increase in new myocytes can be significant and has been shown to improve heart function after myocardial infarction by reducing overall scar mass. Manipulating myocyte proliferation is not limited to a few select microRNAs as direct overexpression of key cell cycle regulators (ie, CDK1+CDK4+CCNB+CCND, cyclin A2 or cyclin D2) or transcription factor proteins (such as knockdown of Meis, overexpression of REST or Tbx20) can also force increases in myocyte proliferation that improve cardiac function after myocardial infarction. 60 , 61 , 62 , 63 , 64 Interestingly, these strategies are not limited to exploiting the cell cycle machinery alone. Overexpression of pyruvate kinase muscle isoenzyme 2, an isoenzyme of the glycolytic enzyme pyruvate kinase, induces cardiomyocyte cell division and improves cardiac function after acute or chronic myocardial infarction. 65
Given that direct in situ stimulation of adult myocyte proliferation is possible, this lends credence to the concept that cell therapy may stimulate myocytes to reenter the cell cycle. Genetic fate mapping studies have shown that intramyocardial injection of HDCs can increase cardiomyocyte cycling by 3‐fold. 66 Several studies panning through the factors secreted directly by these cells (such as stromal cell derived factor 1 alpha 66 ) or extracellular vesicles 36 , 67 have demonstrated biological plausibility. Recently, Balbi and colleagues demonstrated that the extracellular vesicles produced by heart‐derived cells express on their surface a short periostin isoform that appears to mediate the cell cycle activity of these secreted vesicles. 68 How much this enhanced proliferation plays a role in clinical outcomes is uncertain. To date, the only clinical study that has convincingly demonstrated increases in viable myocardium is the CADUCEUS trial where cell‐treated patients had smaller scar sizes, increased viable myocardium, and improved regional function 1 year after treatment. 69 , 70 It remains a challenge for the field to dissect the contribution of myocardial salvage (ie, anti‐apoptotic effects) from true paracrine generation of new cardiomyocytes.
1.4. Progress in paracrine therapies
Recent meta‐analysis of the clinical trials to date suggests that both autologous and allogeneic MSCs are safe with a possible beneficial effect on ejection fraction (SMD 0.73, 95% CI 0.24‐1.21) but no effect on mortality. 3 This discouraging result may not reflect the cell type chosen but rather the timing and manner of delivery. Of the 23 studies included, 12 relied upon intracoronary delivery. 3 Similar results have been seen in clinical data exploring the effectiveness of intracoronary administered autologous hematopoietic cells in ST‐elevation myocardial infarction patients after primary percutaneous coronary intervention. 71 Within these 42 randomized controlled trials, there was no detectable reduction in mortality, arrhythmia, or infarct size and no improvement in quality of life or myocardial function attributable to cell therapy. The authors rightly conclude that intracoronary injection of suspended hematopoietic cells after acute infarction is futile and that future trials would be unlikely to alter this conclusion. The recently completed phase III BAMI trial that administered autologous bone marrow‐derived mononuclear cells to 375 patients after ST elevation myocardial infarction confirmed this prescient warning as cell therapy failed to improve all‐cause mortality, or death/heart failure hospitalization. 72 It is now clear that intracoronary delivery of any cell product that requires a threshold dose to realize therapeutic benefit is unlikely to be effective. In the absence of additional measures designed to enhance cell survival or “dose” of paracrine factors, simply changing paracrine‐dependent cell products is fated to repeat the hematopoietic experience.
Increased understanding that paracrine stimulation underpins therapeutic repair led many in the field to explore the possibility of allogeneic cardiac repair. This approach was predicated on the immune‐evasive properties of many adult cell sources. 73 In the early 2000s, a series of reports began to emerge suggesting that MSCs acted as immune regulators to suppress the proliferation of activated T cells and mixed lymphocyte reactions. 74 This led to the proposal that a large supply of culture‐expanded allogeneic MSCs from a single, universal donor could be expanded to treat all patients. 75 The field of cardiac repair embraced this concept and the immunomodulatory properties of MSCs and related cell types were soon established in preclinical cardiac models. 76 , 77 A series of clinical trials rapidly followed as the allure of an “off the shelf” product was investigated in patients with ischemic cardiomyopathy. 78 , 79 , 80 , 81 To date, the results have been disappointing with no study achieving their primary efficacy outcome. However consistent with the autologous experience, all trials demonstrated that allogeneic cell administration was safe with the generation of anti‐donor antibodies occurring in only a minority of patients (2%‐3%). 78 , 79 , 80 , 81
In part based on these results, several groups have shifted back to exploring autologous paracrine delivery with a focus on new delivery methods and new patient groups. The CardiAMP Heart Failure Trial is one such example where autologous bone marrow mononuclear cells are injected into the myocardium of patients with ischemic cardiomyopathy using a helical delivery catheter. 82 This trial is notable as candidate bone marrow is first screened using a proprietary cell assay that presumably reflects a measure for CD34+ content. Previous work established every 3% increment in CD34+ cells was associated with a ~3% absolute unit increase in cardiac ejection fraction. 82 Qualifying patients then undergo a therapeutic bone marrow biopsy which is processed at the point of care to separate the nucleated cell fraction for immediate transendocardial administration. Reports from the 12‐month feasibility roll in phase have indicated the protocol is safe with promising hints of efficacy. 83
Bone marrow CD34+ cells have also shown promise in other related cardiac diseases. In meta‐analysis and patient level pooled data, transendocardial injection of CD34+ cells to patients with chronic exertional cardiac chest pain reduce both angina frequency and mortality while increasing exercise capacity. 84 , 85 The recent phase II RENEW trial designed to test this concept was terminated prior to completing enrollment by the sponsor for “strategic considerations.” Despite these limitations, the consistency of these results underscores the need for a definitive trial for this promising therapy.
Bone marrow cells are not the only product which has been administered to other cardiac diseases. Heart‐derived CDCs have been administered to patients with pulmonary hypertension (NCT03145298), heart failure with preserved ejection fraction (NCT02941705), Duchenne cardiomyopathy (NCT02485938 & NCT03406780) and congenital single ventricle physiology (NCT01273857 & NCT01829750). The latter being the most interesting application as children with hypoplastic left heart syndrome would likely be most apt to respond to paracrine stimulation of endogenous repair mechanisms. 86 , 87 , 88
1.5. The engraftment hypothesis of cardiac repair
Cell therapy dogma states that long‐term engraftment is needed to realize any benefit from cell transplantation. It follows that, the dose administered dictates the physiologic response as transplanted cells are naturally inclined to survive, engraft, and replace damaged tissue. Over time, several observations have arisen that challenged these underlying presumptions about cell‐mediated repair.
As outline above, the acute retention of any transplanted cell is very modest, yet injured hearts improve after cell injection. Many cells are swept away seconds after intracoronary or intramyocardial injection.
Of those few transplanted cells that are retained, many do not survive for more than a few hours. Days after cell injection only 10% to 20% of the initial injectate can be found and this decreases to 1% to 2% after several weeks, despite clear gains in heart function. Some of this winnowing can be attributed to acute injury that arises from the loss of integrin‐dependent cell signaling experienced during enzymatic harvest for injection (anoikis). Suspension in phosphate‐buffered saline for delivery also limits survival. Cells can also experience significant sheer stresses during delivery as they stream through a needle or catheter. Finally, a portion of cells are lost after delivery into the damaged myocardium replete with inflammatory cells, cytotoxic cytokines, reactive oxygen species and loss of critical matrix attachments. 38
Cell products that are intended to improve heart function by differentiating into working myocardium (ie, PSCs) do not need to provide meaningful increases in new working heart tissue to show therapeutic benefits. 51 , 89 , 90
Increasing the long‐term retention of transplanted cells using biomaterials does not necessarily improve treatment outcomes. Previously, we have shown that encapsulation of heart‐derived cells within protective hydrogel cocoons prior to intramyocardial injection increases acute and long‐term engraftment. 91 , 92 , 93 Akin to many biomaterial studies, increased cell retention was associated with salutary effects on myocardial function and scar size. Dogma would traditionally interpret this result as proof that increased cell retention leads to better heart function. However, we found that reducing cocoon density decreased the paracrine repertoire of these cocooned cells. Cells with reduced cocoon density had identical short and long‐term engraftment but, because of their reduced paracrine output, treatment effects were similar to intramyocardial injection of non‐cocooned cells. Thus, treatment effects depended solely on the paracrine product produced by these cells and had little relation to cell retention.
In pharmacology, a monotonic gradient exists whereby increased drug exposure results in increased physiological effect. To date, there are no convincing studies demonstrating a biological gradient to support a causal association between long‐term cell retention and an improvement in heart function.
As such, a more nuanced view of cardiac cell engraftment has begun to emerge. 94 The data supports the view that early cell retention predicts transplant outcomes, and some degree of retention is likely important. Much of the biomaterial work directed toward increasing cell retention likely modifies the paracrine output of transplanted cells to improve treatment effects. When the paracrine output of a cell product is fixed and unchanging, there is likely a “threshold dose” of transplanted cells that once attained, further increases in cell number are not needed or beneficial. Transplant outcomes become dependent on increasing exposure to a more potent paracrine cell product rather than simply increasing the number of cells retained. It follows that a highly potent paracrine product will evoke a given improvement in cardiac function after a low number of cells have been retained, while a cell product of lower potency will evoke the same response only after a greater number of cells have been retained. In the coming years, these observations will likely play important roles in directing the progress of the field by defining how and when a cell therapy is administered to patients.
2. PROGRESS TOWARD MYOCYTE REPLACEMENT
With the advent of new cellular reprogramming techniques, several groups have focused on restoring cardiac function by directly replacing lost myocytes with new cardiomyocytes derived from PSCs. Unlike paracrine‐based therapies, this strategy is inherently tied to successful engraftment and long‐term retention of functional cardiomyocytes. 95
Early work naturally centered on perfecting techniques to direct differentiation of PSCs toward a cardiac lineage. Contemporary protocols now commonly involve using small molecules to modulate the canonical Wnt signaling pathway to first promote mesoderm specification through activation of Wnt followed by specification to a cardiac progenitor identity by inhibiting Wnt. 96 , 97 A variety of selection strategies can then be used to yield a 95%+ population of beating cardiomyocytes. 98
Despite this progress, the field continues to struggle with problems related to maturation of PSC‐derived cardiomyocytes. These immature cells differ from adult cardiomyocytes in their contractility, electrophysiology, metabolism, morphology and response to calcium or β‐adrenergic stimulation. 99 In response, many groups have looked toward customized small molecules, extracellular matrix scaffolds, and electrical/mechanical stimuli to improve tissue maturation and function with some success. But these hurdles are not inconsequential as regulatory approval will likely require that an eventual PSC‐derived cell therapy fully address all issues related to arrhythmogenicity, immunogenicity and tumorigenicity. 100 As outlined above, early trials are now underway to investigate the ability of hydrogel patches or stacked cell sheets to improve function and establish product safety. For example, the recently started BioVAT‐HF Trial (NCT04396899) combines PSC‐derived cardiomyocytes with stromal cells in a bovine collagen type I hydrogen to enhance improve heart function in patients with end‐stage heart failure. This interesting approach leverages multiple mechanisms to enhance heart function, namely complimentary cell types with possible additive benefits while the biomaterial will provide mechanical support to the damaged myocardium, limit anoikis of transplanted cells and restore extracellular matrix cues lost during post infarct remodeling. Given concerns regarding pro‐arrhythmia, many trials have so far been restricted to patients with an implantable cardioverter defibrillator in place to abort sudden cardiac death and most have prophylactically treated patients with antiarrhythmic medications to prevent initiation of cardiac arrhythmias. Reassuringly, a recent small (n = 6) open label phase 1 trial of embryonic stem cell‐derived CD15+ Isl‐1 progenitor cells demonstrated the technical feasibility and 12‐month safety of this approach 101 ; thus, rationalizing future, adequately powered, efficacy studies in this exciting area.
3. CONCLUSION AND FUTURE DIRECTIONS
The last two decades have provided a wealth of insights into the mechanics and mechanisms of cardiac regeneration. Clearly, the human heart possesses low level turn over as tissue is dismantled, destroyed, and rebuilt. Exogenous adult and pluripotent cells can stimulate repair, but these benefits have limits which reflect the cell source potency and recipient capacity. Given that paracrine factors recapitulate many of the effects seen with parent cells, recent work has naturally focused on the extracellular vesicles secreted by these cells, or the defined factors contained within these vesicles. This innovation has the potential to “bypass” paracrine cell injection and, unlike a systemic drug that binds to a single receptor, extracellular vesicles or defined factors can be selectively uptaken by target cells (which reduces off target side effects) and engineered to exploit many different complimentary pathways (unlike a drug that stimulates a single response). Live cell administration will still have a role as a scar replacement source, but these products will need to establish a robust safety profile to increase feasibility and the interplay between these two technologies remains to be defined over the coming years.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
R.V.: conception and design, manuscript writing, final approval of manuscript; D.R.D.: conception and design, financial support, manuscript writing, final approval of manuscript.
Supporting information
Appendix S1. Supporting information
Supplementary Figure 1. PRISMA flow diagram for literary search
ACKNOWLEDGMENTS
We thank Sarah Visintini MLIS (Berkman Library, University of Ottawa Heart Institute) for conducting literature searches in support of this project. This work was supported by the Canadian Institutes of Health Research (Project Grant 410103, Collaborative Health Research Project 433391).
Vaka R, Davis DR. State‐of‐play for cellular therapies in cardiac repair and regeneration. Stem Cells. 2021;39(12):1579‐1588. doi: 10.1002/stem.3446
Funding information Institute of Circulatory and Respiratory Health, Grant/Award Number: 410103
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. World Health Organization . The Top 10 Causes of Death; 2021. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed March 12, 2021.
- 2. Bloom DE, Cafiero ET, Janeané‐Llopis E, et al. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011. [Google Scholar]
- 3. Lalu MM, Mazzarello S, Zlepnig J, et al. Safety and efficacy of adult stem cell therapy for acute myocardial infarction and ischemic heart failure (SafeCell Heart): a systematic review and meta‐analysis. Stem Cells Translational Medicine. 2018;7:857‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Povsic TJ, Gersh BJ. Stem cells in cardiovascular diseases: 30,000‐foot view. Cells. 2021;10:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curfman G. Stem cell therapy for heart failure: an unfulfilled promise? JAMA. 2019;321:1186‐1187. [DOI] [PubMed] [Google Scholar]
- 6. Madonna R, Van Laake LW, Davidson SM, et al. Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell‐based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J. 2016;37:1789‐1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konstantinov IE. In search of Alexander A. Maximow: the man behind the unitarian theory of hematopoiesis. Perspect Biol Med. 2000;43:269‐276. [DOI] [PubMed] [Google Scholar]
- 8. Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452‐454. [DOI] [PubMed] [Google Scholar]
- 9. Gatti RA, Meuwissen HJ, Allen HD, et al. Immunological reconstitution of sex‐linked lymphopenic immunological deficiency. Lancet. 1968;2:1366‐1369. [DOI] [PubMed] [Google Scholar]
- 10. Bortin MM. A compendium of reported human bone marrow transplants. Transplantation. 1970;9:571‐587. [DOI] [PubMed] [Google Scholar]
- 11. Santos GW, Sensenbrenner LL, Burke PJ, et al. Marrow transplantation in man following cyclophosphamide. Transplant Proc. 1971;3:400‐404. [PubMed] [Google Scholar]
- 12. Thomas ED, Storb R, Clift RA, et al. Bone‐marrow transplantation (second of two parts). N Engl J Med. 1975;292:895‐902. [DOI] [PubMed] [Google Scholar]
- 13. Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977;49:511‐533. [PubMed] [Google Scholar]
- 14. Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701‐705. [DOI] [PubMed] [Google Scholar]
- 16. Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763‐776. [DOI] [PubMed] [Google Scholar]
- 17. Leri A, Rota M, Hosoda T, et al. Cardiac stem cell niches. Stem Cell Res. 2014;13:631‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tallini YN, Greene KS, Craven M, et al. c‐kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jesty SA, Steffey MA, Lee FK, et al. c‐kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA. 2012;109:13380‐13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaruba MM, Soonpaa M, Reuter S, et al. Cardiomyogenic potential of C‐kit(+)‐expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Berlo JH, Kanisicak O, Maillet M, et al. c‐kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sultana N, Zhang L, Yan J, et al. Resident c‐kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hatzistergos KE, Takeuchi LM, Saur D, et al. cKit+ cardiac progenitors of neural crest origin. Proc Natl Acad Sci USA. 2015;112:13051‐13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Yang R, Huang X, et al. Genetic lineage tracing identifies in situ Kit‐expressing cardiomyocytes. Cell Res. 2016;26:119‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eschenhagen T, Bolli R, Braun T, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136:680‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cheng K, Ibrahim A, Hensley MT, et al. Relative roles of CD90 and c‐Kit to the regenerative efficacy of cardiosphere‐derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc. 2014;3:e001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheng K, Shen D, Sun B, et al. Irrelevance of c‐kit‐positive subpopulation to the therapeutic benefit of human cardiosphere‐derived cells. Circulation. 2012;126:A13259. [Google Scholar]
- 28. Rafatian G, Davis DR. Concise review: heart‐derived cell therapy 2.0: paracrine strategies to increase therapeutic repair of injured myocardium. Stem Cells. 2018;36:1794‐1803. [DOI] [PubMed] [Google Scholar]
- 29. Davis DR. Cardiac stem cells in the post‐Anversa era. Eur Heart J. 2019;40:1039‐1041. [DOI] [PubMed] [Google Scholar]
- 30. Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911‐921. [DOI] [PubMed] [Google Scholar]
- 31. White AJ, Smith RR, Matsushita S, et al. Intrinsic cardiac origin of human cardiosphere‐derived cells. Eur Heart J. 2011;1:68‐75. [DOI] [PubMed] [Google Scholar]
- 32. Davis DR, Kizana E, Terrovitis J, et al. Isolation and expansion of functionally‐competent cardiac progenitor cells directly from heart biopsies. JMCC. 2010;49:312‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li T‐S, Lee S‐T, Matsushita S, et al. Cardiospheres recapitulate a niche‐like microenvironment rich in stemness and cell‐matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;105:2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS ONE. 2009;4:e7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere‐derived cells transplanted into infarcted mice. Circ Res. 2010;106:971‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014;2:606‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere‐derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896‐908. [DOI] [PubMed] [Google Scholar]
- 38. Kanda P, Davis DR. Cellular mechanisms underlying cardiac engraftment of stem cells. Expert Opin Biol Ther. 2017;17:1127‐1143. [DOI] [PubMed] [Google Scholar]
- 39. Mount S, Davis DR. Electrical effects of stem cell transplantation for ischaemic cardiomyopathy: friend or foe? J Physiol. 2016;594:2511‐2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159‐167. [DOI] [PubMed] [Google Scholar]
- 41. Bolli R, Hare JM, March KL, et al. Rationale and design of the CONCERT‐HF trial (combination of mesenchymal and c‐kit(+) cardiac stem cells as regenerative therapy for heart failure). Circ Res. 2018;122:1703‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hartung T. Look back in anger—what clinical studies tell us about preclinical work. ALTEX. 2013;30:275‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Forum on Neuroscience and Nervous System Disorders . Board on Health Sciences Policy; Institute of Medicine. Improving and Accelerating Therapeutic Development for Nervous System Disorders: Workshop Summary. Drug Development Challenges. Washington, DC: National Academies Press (US); 2014. [PubMed] [Google Scholar]
- 44. Hingorani AD, Kuan V, Finan C, et al. Improving the odds of drug development success through human genomics: modelling study. Sci Rep. 2019;9:18911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Terrovitis J, LautaμSki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac‐derived stem cell delivery. J Am Col Cardiol. 2009;54:1619‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Terrovitis J, Kwok KF, Lautamaki R, et al. Ectopic expression of the sodium‐iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single‐photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bonios M, Terrovitis J, Chang CY, et al. Myocardial substrate and route of administration determine acute cardiac retention and lung bio‐distribution of cardiosphere‐derived cells. J Nucl Cardiol. 2011;18:443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Terrovitis J, Lautamäki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac‐derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lima Correa B, El Harane N, Gomez I, et al. Extracellular vesicles from human cardiovascular progenitors trigger a reparative immune response in infarcted hearts. Cardiovasc Res. 2021;117:292‐307. [DOI] [PubMed] [Google Scholar]
- 50. El Harane N, Kervadec A, Bellamy V, et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J. 2018;39:1835‐1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kervadec A, Bellamy V, El Harane N, et al. Cardiovascular progenitor‐derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant. 2016;35:795‐807. [DOI] [PubMed] [Google Scholar]
- 52. Jiang X, Yang Z, Dong M. Cardiac repair in a murine model of myocardial infarction with human induced pluripotent stem cell‐derived cardiomyocytes. Stem Cell Res Ther. 2020;11:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saad AM, Abushouk AI, Al‐Husseini MJ, et al. Characteristics, survival and incidence rates and trends of primary cardiac malignancies in the United States. Cardiovasc Pathol. 2018;33:27‐31. [DOI] [PubMed] [Google Scholar]
- 54. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science (New York, NY). 2009;324:98‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hsieh PC, Segers VF, Davis ME, et al. Evidence from a genetic fate‐mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Senyo SE, Steinhauser ML, Pizzimenti CL, et al. Mammalian heart renewal by pre‐existing cardiomyocytes. Nature. 2013;493:433‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hesse M, Raulf A, Pilz GA, et al. Direct visualization of cell division using high‐resolution imaging of M‐phase of the cell cycle. Nat Commun. 2012;3:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borden A, Kurian J, Nickoloff E, et al. Transient introduction of miR‐294 in the heart promotes cardiomyocyte cell cycle reentry after injury. Circ Res. 2019;125:14‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tian Y, Liu Y, Wang T, et al. A microRNA‐Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015;7:279ra238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng RK, Asai T, Tang H, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741‐1748. [DOI] [PubMed] [Google Scholar]
- 61. Pasumarthi KB, Nakajima H, Nakajima HO, et al. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110‐118. [DOI] [PubMed] [Google Scholar]
- 62. Mohamed TMA, Ang YS, Radzinsky E, et al. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell. 2018;173:104‐116. e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang D, Wang Y, Lu P, et al. REST regulates the cell cycle for cardiac development and regeneration. Nat Commun. 2017;8:1979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64. Xiang FL, Guo M, Yutzey KE. Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation. 2016;133:1081‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Magadum A, Singh N, Kurian AA, et al. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation. 2020;141:1249‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malliaras K, Ibrahim A, Tseliou E, et al. Stimulation of endogenous cardioblasts by exogenous cell therapy after myocardial infarction. EMBO Mol Med. 2014;6:760‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Barile L, Lionetti V, Cervio E, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530‐541. [DOI] [PubMed] [Google Scholar]
- 68. Balbi C, Milano G, Fertig TE, et al. An exosomal‐carried short periostin isoform induces cardiomyocyte proliferation. Theranostics. 2021;11:5634‐5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Makkar RR, Smith RR, Cheng K, et al. Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Malliaras K, Makkar RR, Smith RR, et al. Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63:110‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jeyaraman MM, Rabbani R, Copstein L, et al. Autologous bone marrow stem cell therapy in patients with ST‐elevation myocardial infarction: a systematic review and meta‐analysis. Can J Cardiol. 2017;33:1611‐1623. [DOI] [PubMed] [Google Scholar]
- 72. Mathur A, Fernández‐Avilés F, Bartunek J, et al. The effect of intracoronary infusion of bone marrow‐derived mononuclear cells on all‐cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J. 2020;41:3702‐3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T‐cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389‐397. [DOI] [PubMed] [Google Scholar]
- 75. Klyushnenkova E, Mosca JD, Zernetkina V, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47‐57. [DOI] [PubMed] [Google Scholar]
- 76. Huang XP, Sun Z, Miyagi Y, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long‐term benefits for myocardial repair. Circulation. 2010;122:2419‐2429. [DOI] [PubMed] [Google Scholar]
- 77. Malliaras K, Li TS, Luthringer D, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere‐derived cells. Circulation. 2012;125:100‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA. 2012;308:2369‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Makkar RR, Kereiakes DJ, Aguirre F, et al. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): a randomized, placebo‐controlled, double‐blinded trial. Eur Heart J. 2020;41:3451‐3458. [DOI] [PubMed] [Google Scholar]
- 80. Li X, Hu YD, Guo Y, et al. Safety and efficacy of intracoronary human umbilical cord‐derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr Pharm Des. 2015;21:1426‐1432. [DOI] [PubMed] [Google Scholar]
- 81. Zhao XF, Xu Y, Zhu ZY, et al. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015;14:3010‐3017. [DOI] [PubMed] [Google Scholar]
- 82. Raval AN, Cook TD, Duckers HJ, et al. The CardiAMP Heart Failure trial: a randomized controlled pivotal trial of high‐dose autologous bone marrow mononuclear cells using the CardiAMP cell therapy system in patients with post‐myocardial infarction heart failure: trial rationale and study design. Am Heart J. 2018;201:141‐148. [DOI] [PubMed] [Google Scholar]
- 83. Raval AN, Johnston PV, Duckers HJ, et al. Point of care, bone marrow mononuclear cell therapy in ischemic heart failure patients personalized for cell potency: 12‐month feasibility results from CardiAMP heart failure roll‐in cohort. Int J Cardiol. 2021;326:131‐138. [DOI] [PubMed] [Google Scholar]
- 84. Velagapudi P, Turagam M, Kolte D, et al. Intramyocardial autologous CD34+ cell therapy for refractory angina: a meta‐analysis of randomized controlled trials. Cardiovasc Revasc Med. 2019;20:215‐219. [DOI] [PubMed] [Google Scholar]
- 85. Henry TD, Losordo DW, Traverse JH, et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no‐option refractory angina: a patient‐level pooled analysis of randomized double‐blinded trials. Eur Heart J. 2018;39:2208‐2216. [DOI] [PubMed] [Google Scholar]
- 86. Tarui S, Ishigami S, Ousaka D, et al. Transcoronary infusion of cardiac progenitor cells in hypoplastic left heart syndrome: three‐year follow‐up of the Transcoronary Infusion of Cardiac Progenitor Cells in Patients With Single‐Ventricle Physiology (TICAP) trial. J Thorac Cardiovasc Surg. 2015;150:1198‐1207, 1208 e1191‐1192. [DOI] [PubMed] [Google Scholar]
- 87. Ishigami S, Ohtsuki S, Tarui S, et al. Intracoronary autologous cardiac progenitor cell transfer in patients with hypoplastic left heart syndrome: the TICAP prospective phase 1 controlled trial. Circ Res. 2015;116:653‐664. [DOI] [PubMed] [Google Scholar]
- 88. Ishigami S, Ohtsuki S, Eitoku T, et al. Intracoronary cardiac progenitor cells in single ventricle physiology: the PERSEUS (cardiac progenitor cell infusion to treat univentricular heart disease) randomized phase 2 trial. Circ Res. 2017;120:1162‐1173. [DOI] [PubMed] [Google Scholar]
- 89. Tachibana A, Santoso MR, Mahmoudi M, et al. Paracrine effects of the pluripotent stem cell‐derived cardiac myocytes salvage the injured myocardium. Circ Res. 2017;121:e22‐e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhu K, Wu Q, Ni C, et al. Lack of remuscularization following transplantation of human embryonic stem cell‐derived cardiovascular progenitor cells in infarcted nonhuman primates. Circ Res. 2018;122:958‐969. [DOI] [PubMed] [Google Scholar]
- 91. Mayfield AE, Tilokee EL, Latham N, et al. The effect of encapsulation of cardiac stem cells within matrix‐enriched hydrogel capsules on cell survival, post‐ischemic cell retention and cardiac function. Biomaterials. 2014;35:133‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kanda P, Benavente‐Babace A, Parent S, et al. Deterministic paracrine repair of injured myocardium using microfluidic‐based cocooning of heart explant‐derived cells. Biomaterials. 2020;247:120010. [DOI] [PubMed] [Google Scholar]
- 93. Kanda P, Alarcon EI, Yeuchyk T, et al. Deterministic encapsulation of human cardiac stem cells in variable composition nanoporous gel cocoons to enhance therapeutic repair of injured myocardium. ACS Nano. 2018;12:4338‐4350. [DOI] [PubMed] [Google Scholar]
- 94. Davis DR. The cell engraftment hypothesis of cardiac repair. Curr Stem Cell Res Ther. 2020;15:711‐722. [DOI] [PubMed] [Google Scholar]
- 95. Matsuo T, Masumoto H, Tajima S, et al. Efficient long‐term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci Rep. 2015;5:16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ueno S, Weidinger G, Osugi T, et al. Biphasic role for Wnt/beta‐catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci USA. 2007;104:9685‐9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burridge PW, Keller G, Gold JD, et al. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell. 2012;10:16‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Paik DT, Chandy M, Wu JC. Patient and disease‐specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol Rev. 2020;72:320‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Neofytou E, O'Brien CG, Couture LA, et al. Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest. 2015;125:2551‐2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Menasche P, Vanneaux V, Hagege A, et al. Transplantation of human embryonic stem cell‐derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. 2018;71:429‐438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Supplementary Figure 1. PRISMA flow diagram for literary search
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


