Abstract
Background
Congenital melanocytic naevi (CMN) can have a great impact on patients’ lives owing to perceived stigmatization, and the risk of melanoma development and neurological complications. Development of a core outcome set (COS) for care and research in CMN will allow standard reporting of outcomes. This will enable comparison of outcomes, allowing professionals to offer advice about the best management options. In previous research, stakeholders (patients, parents and professionals) reached consensus on the core domains of the COS. To select the appropriate measurement instruments, the domains should be specified by outcomes.
Objectives
To reach consensus on the specific core outcomes describing the core domains pertaining to clinical care and research in CMN.
Methods
A list of provisional outcomes (obtained earlier) was critically reviewed by the Outcomes for COngenital MElanocytic Naevi (OCOMEN) research team and by relevant stakeholders through an online questionnaire, to refine this list and provide clear definitions for every outcome. When needed, discussion with individual participants was undertaken over the telephone or by email. During an online consensus meeting, stakeholders discussed the inclusion of potential outcomes. After the meeting, participants voted in two rounds for the inclusion of outcomes.
Results
Forty‐four stakeholders from 19 countries participated. Nine core outcomes were included in the COS relative to clinical care and 10 core outcomes for research.
Conclusions
These core outcomes will enable standard reporting in future care and research of CMN. This study facilitates the next step of COS development: selecting the appropriate measurement instruments for every outcome.
Short abstract
What is already known about this topic?
Congenital melanocytic naevi (CMN) can be associated with psychosocial burden and increased risk of melanoma and/or neurological complications.
Outcomes measured for research and care in CMN are heterogeneous, impeding comparison.
A core outcome set (COS) may enhance standardized use and reporting, and reduce selective reporting bias.
In previous research, relevant stakeholders reached consensus on what domains should be included in the core domain set (CDS).
What does this study add?
To select the appropriate measurement instruments for the domains included in the CDS, the domains should be further specified by outcomes.
We reached consensus on what outcomes should describe the domains of the CDS of CMN care and research.
Through a consensus procedure, including online discussions, online consensus meeting and voting, relevant stakeholders reached consensus on a limited number of core outcomes describing the core domains.
What are the clinical implications of this work?
Development of a COS will allow standard reporting of outcomes in future care and research of CMN.
This will enable pooling and comparison of outcomes, allowing guideline development of optimal management policy.
Linked Comment: M.V. Heppt et al. Br J Dermatol 2021; 185:881–882.
Plain language summary available online
Owing to their unusual appearance, congenital melanocytic naevi (CMN) can have a great impact on patients’ lives. 1 In addition, people with CMN with large or giant [> 20 cm projected adult size (PAS)], or multiple CMN have an elevated risk of developing melanoma and neurological complications. 2 , 3 Comparison of management strategies is currently hindered by the lack of standard and uniform outcome reporting. 4 , 5 This impedes guidance on the optimal management policy based on high‐evidence research. 6 To address this problem, a core outcome set (COS) needs to be developed, i.e. a consensus‐derived minimum set of outcomes that should be measured and reported in all care of or clinical research concerning a certain health condition. 7 Ideally, a COS describes both what should be measured (domains and outcomes) and how this should be measured (measurement instruments). 7 The Outcomes for COngenital MElanocytic Naevi (OCOMEN) project aims to develop a COS for CMN. Currently, the project is focused on the ‘what’ to measure. The first step was performed in 2020, i.e. consensus was reached on which domains should be included in the core domain set (CDS) of care of, and research on, CMN. 8
In this study, we define ‘domains and outcomes’ as aspects of disease that could be measured to evaluate different management strategies. ‘Domains’ are broader aspects of a disease like ‘neoplasm’, whereas ‘outcomes’ are defined as more precise aspects of a disease on a lower hierarchical level, for example ‘presence of melanoma’ is an outcome on a lower hierarchical level of the domain ‘neoplasm’. We define a ‘baseline characteristic’ as a demographic, clinical or prognostic aspect of the patient, like age, sex or the location of their CMN. To be able to select the right measurement instrument the domains should be defined in terms of measurable specific outcomes.
Table S1 (see Supporting Information) shows the list of domains and provisional outcomes obtained in previous research. 8 To promote future uptake of the CMN CDS, only a limited set of core outcomes should be measured. These are the ones agreed to be most necessary to measure and report in all future care or research concerning CMN management. A limited number of outcomes makes the CDS feasible to use in daily practice and research. Of course, researchers and professionals could always measure additional outcomes that they deem relevant.
The aim of this study was to reach consensus on outcomes describing the core domains that are most necessary to measure and report in all future care or research encompassed by the core domains.
Materials and methods
Scope and applicability of the core outcome set
The target population is patients with medium‐to‐giant CMN: patients with a CMN of 1·5 cm in diameter or larger PAS on the face, and CMN > 10 cm diameter PAS elsewhere on the body. 9 The COS is intended for use in all types of interventions of CMN: interventional management (excision, laser, curettage and dermabrasion), as well as conservative management (watchful waiting). Distinct COS are being developed for care (clinical practice) and for research (clinical observational studies and trials), each for international use. 10
Ethical approval was obtained from the Ethical Review Board at Erasmus MC Rotterdam and from the Ethical Board at Amsterdam UMC. The OCOMEN project was registered in the Core Outcome Measures in Effectiveness Trials (COMET) database. We followed the guidelines of the COMET initiative, 7 , 11 , 12 and the Cochrane Skin‐Core Outcomes Set Initiative (CS‐COUSIN). 13 We reported this article according to the Core Outcome Set – STAndards for Development (COS‐STAD) and Core Outcome Set – STAndards for Reporting (COS‐STAR). 12 , 14 The protocols of consensus meetings of other COS research groups were used for the CMN consensus meeting. 15 , 16 A protocol of the consensus procedure was sent to CS‐COUSIN, who provided methodological feedback. 17 The participants were asked for their consent to publish their names in the acknowledgments. None of the participants objected (see also Table S2; Supporting Information).
Table 1 shows the ways in which we involved relevant stakeholders, including patients, parents and health professionals in this project.
Table 1.
Stakeholder groups and methods of approaching potential participants
| Stakeholder groups | Details | Approach methods |
|---|---|---|
| Patients/parents | Patients; parents/caregivers;a family members | Identified patients who participated in previous research of the OCOMEN project |
| Call on social media and patient support organization websites for participation (˜4000 views) | ||
| Collaboration with national and international patient advocates who used their network to invite patients | ||
| Call for participants at the Naevus Global patient representative meeting (12 September 2019) | ||
| Professionals | Dermatologists; plastic surgeons; pathologists; neurologists; psychologists; researchers | Identified professionals who participated in previous OCOMEN project research |
| Identification of names from the literature, attendance at meetings/conferences about paediatric dermatology/plastic surgery and through personal network of the OCOMEN team | ||
| Participants were asked to suggest names of other professionals who may be interested in participating |
OCOMEN, Outcomes for COngenital MElanocytic Naevi. aParents could complete the survey based on their own personal perspective or on behalf of their young child, in which case they needed to do the rating based on the child’s perspective.
Finding the core outcomes that describe the core domains involved four steps: (i) reviewing the provisional list of outcomes by the OCOMEN team (i.e. defining and reordering outcomes); (ii) reviewing the provisional list of outcomes with relevant stakeholders; (iii) an online consensus meeting with relevant stakeholders to discuss the inclusion/exclusion of outcomes that are core and most necessary to measure and report in all future care or research; and (iv) voting for inclusion/exclusion of outcomes, resulting in a list of outcomes per domain.
Reviewing the provisional list of outcomes by the Outcomes for Congenital Melanocytic Naevi team
The provisional list of outcomes (list 1; Table S1) was obtained in previous research that aimed to reach consensus on the core domains. In this research, participants could give their provisional vote on which outcomes should describe the domains during the last round of an e‐Delphi study and during a consensus meeting. 8 These outcomes were obtained through a previously performed systematic review and focus groups. 5 , 10
We aimed to prepare this provisional list of outcomes to enable an easier discussion of inclusion and exclusion of outcomes during the consensus meeting. The OCOMEN team critically reviewed the following aspects for every separate outcome of list 1: (i) Is the name and definition of the outcome clear? (ii) Was the outcome classified in the right domain? (3) Is it an outcome and not a baseline characteristic like age, sex or body location of the CMN? (4) Could the outcome be lumped together with another outcome? A clinical psychologist (L.H.) was consulted to review the outcomes concerning ‘quality of life’. Definitions were provided in order to make the outcomes clear for all participants. We used the Patient Reported Outcomes Measurement Information System (PROMIS) definitions for the definitions of the outcomes of the ‘quality of life’ domain. 18 , 19 Two parents of children with CMN were consulted to ensure that all outcomes and definitions were written in lay language. These alterations resulted in list 2: ‘provisional list of outcomes reviewed by the OCOMEN team’ (Table S3; see Supporting Information).
Reviewing the provisional list of outcomes with relevant stakeholders
We asked relevant stakeholders to give their feedback on list 2 (Table S3). They were sent an online questionnaire, where they could state their opinion. When needed, discussion was performed by email or telephone. One week before the consensus meeting, participants received list 3 [‘provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders’ (Table S4; see Supporting Information)]. When participants disagreed with alterations made, they were offered an opportunity to discuss this with one of the OCOMEN team members by telephone before the consensus meeting, to ensure that everyone’s opinion was heard. Participants did not have the opportunity to discuss these alterations during the consensus meeting. This deviation of the protocol was made to shorten the discussion during the online consensus meeting by only discussing the inclusion/exclusion of outcomes.
Online consensus meeting to discuss inclusion/exclusion of outcomes
An online consensus meeting was held on 21 January 2020 via Zoom (https://zoom.us), with the aim of discussing the inclusion and exclusion of potential outcomes. Patients, parents and professionals specialized in CMN were able to participate in this meeting (Table S2). A meeting protocol with a timetable was made beforehand, to ensure that all outcomes could be discussed in the 2‐h consensus meeting.
Before the meeting, participants received the following information: (i) what a COS is vs. a CDS and why they are necessary; 7 (ii) information about previously conducted research in CDS development; 5 , 8 , 10 (iii) the list of domains included in the CDS for care and research; (iv) the provisional list of outcomes per domain; (v) the list of outcomes excluded during the Delphi round and consensus meeting for the CDS in 2019; (vi) a definition list of outcomes in lay language for patients who participated; (vii) explanation of what should be expected of the meeting; and (viii) explanation that when an outcome is included in the CDS, it should always be measured in all care or all research.
The consensus meeting started with personal introductions from all participants, which was followed by a presentation repeating the information listed above. After the presentation we started a plenary whole‐group discussion. An audio discussion was moderated by one of the OCOMEN team members. In the meantime, there was a written chat discussion moderated by another OCOMEN team member. We discussed whether the domain ‘adverse event’ should be included in the CDS of research, because it was only included in the CDS of care. Subsequently, we discussed each outcome of list 3 (‘provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders’; Table S4).
Voting for inclusion/exclusion of outcomes, resulting in a list of outcomes per domain
The voting was done in two rounds to enable participants to see the first judgement of the group after the first round of voting and revise their earlier answers in light of the replies of other participants. The first round was performed at the end of the consensus meeting by the anonymous voting option in Zoom. The second vote was the final round of voting and was done via email. Participants who attended the consensus meeting received a Microsoft Word document with results of the first voting. In this document they could vote for which outcomes they considered to be core. When at least 70% of participants considered an outcome to be core in the final voting, the outcome was included in the CDS.
Results
Participants
We initially contacted 193 stakeholders, of whom 79 were initially interested in participating. Forty‐four stakeholders participated in the study, i.e. gave feedback on the list of outcomes (n = 33) and/or participated in the consensus meeting (n = 32). Twelve of the participants who gave their feedback on the provisional list of outcomes were not able to attend the meeting and 11 participants who attended the meeting did not gave their feedback on the provisional list of outcomes. A total of 30 participants gave their final vote for the care setting and 31 participants for the research setting. Table S2 shows the details of the participants from 19 different countries.
Reviewing the provisional list of outcomes
The OCOMEN team critically reviewed the 28 outcomes of list 1 (‘provisional list of outcomes’; Table S1). The alterations made are shown in list 2 (‘a provisional list of outcomes reviewed by the OCOMEN team’; Table S3). A total of 33 participants gave feedback on list 2 (Table S3).
Based on the participants’ feedback, alterations were made to prepare the list of outcomes for the consensus meeting (list 3: ‘provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders’; Table S4). Definitions of lists 2 and 3 are given in Appendix S1 (see Supporting Information).
Online consensus meeting to discuss inclusion/exclusion of outcomes
A total of 32 stakeholders participated in the consensus meeting. We first discussed whether the domain ‘adverse events’ should be included in both the care and research CDS. In previous research, this domain was excluded for the research CDS. All participants agreed that ‘adverse events’ is a core domain for research and was therefore also included in the research CDS.
Some outcomes that were discussed during the consensus meeting could be interpreted as both an outcome (i.e. aspects to evaluate change by management) or as a baseline characteristic (i.e. demographics, clinical or prognostic aspects). For instance, size, colour, texture or number of CMN can be considered to be baseline characteristics by which to classify the CMN. These baseline characteristics are important in estimating the risk of melanoma or neurological involvement. However, these aspects of the appearance of the CMN can also be used to evaluate different types of management. For instance, the ‘size of CMN’ can reduce after (partial) excision; the ‘colour of CMN’ can lighten after laser treatment; the ‘texture of CMN’ can change as a result of scarring; and ‘satellite naevi number’ can increase during watchful waiting in the first years of life. After discussion with the entire group, we decided that these mutable aspects themselves should be considered as outcomes.
In our previous report, the outcome ‘ability to cope’ was classified as a core outcome for CMN. This outcome is also considered to be core in other conditions like bereavement support in palliative care. 20 We explained that ‘ability to cope’ is something that is influenced by personality and learned coping strategies, and not by the usual direct treatments of CMN (surgery or watchful waiting). Therefore, ‘ability to cope’ is a difficult outcome to use to evaluate the usual treatments of CMN. It could be used as a baseline characteristic to predict other outcomes like ‘emotional distress’ or ‘satisfaction with treatment’. During the group discussion, it was suggested that ‘ability to cope with stigmatization’ could be influenced by treatment such as complete excision of CMN. However, we agreed that the outcome measured in this case would be ‘stigmatization’ and not the ‘ability to cope’.
The most serious clinical threats of CMN are the development of melanoma and neurological complications. Consequently, most participants agreed that these outcomes ‘presence of melanoma’ and ‘neurological symptoms and signs’ are most necessary to measure for both care and research. Therefore, in all future CMN studies, the presence of melanoma or neurological symptoms/signs need to be explicitly documented.
The outcome ‘molecular characteristics’ was proposed during the consensus. Molecular characteristics can be assessed to evaluate (malignant) changes in the CMN tissue. 21 , 22 , 23 Research on COS uptake emphasized that outcomes should be feasible to measure (easily measurable and requiring minimal resources) and responsive to interventions. 24 Testing molecular characteristics could be costly and may hinder researchers in measuring this outcome as standard. Moreover, a skin biopsy is needed to investigate malignancy, which can be a burden for patients in the absence of a clinical rationale. Therefore, we decided with the consensus of the whole group that ‘molecular characteristics’ should be reported in research when such tests were already performed for clinical care. This will increase the standard publication of ‘molecular characteristics’ found in patients with CMN and will improve knowledge on this topic.
Voting for inclusion/exclusion of outcomes, resulting in a list of outcomes per domain
A total of 22 participants voted in the first round at the end of the consensus meeting. Ten participants did not vote because they had to leave the meeting before the end (n = 7), had a bad internet connection (n = 1), had expertise in only one domain (n = 1) or voted together with another participant (n = 1).
Of the 32 people who participated in the consensus meeting, the final vote was made by 30 participants for the care setting and by 31 for the research setting. One participant only voted for research as this participant was only involved in research of CMN. Another participant in the consensus meeting had expertise about only one domain and therefore chose not to vote. Table 2 shows the reviewed provisional list of outcomes and the results of voting in the first and second rounds. Figure 1 shows the list of core outcomes per domain for care and research. The following core outcomes were included in both the CDS of care and research: satellite naevi number; colour of the CMN; texture of the CMN; size of CMN; emotional distress; presence of melanoma; neurological symptoms and signs; wound problems in the CMN; and scar problems. In the research CDS, the outcome ‘molecular characteristics’ was also included.
Table 2.
Reviewed list of provisional outcomes per domain and voting results
|
COS of care domains and provisional outcomes |
Care voting round 1 (n = 22) |
Care voting round 2 (n = 30) |
Research voting round 1 (n = 22) |
Research voting round 2 (n = 31) |
|---|---|---|---|---|
| 1. Anatomy of skin | ||||
| Satellite naevi number | 21 (95) | 26 (87) | 21 (95) | 30 (97) |
| Colour of the CMN | 20 (91) | 22 (73) | 19 (86) | 23 (74) |
| Texture of the CMN | 20 (91) | 25 (83) | 19 (86) | 24 (77) |
| Size of CMN | 20 (91) | 28 (93) | 19 (86) | 25 (81) |
| Hairiness of the CMN | 19 (86) | 17 (57) | 19 (86) | 17 (55) |
| 2a. Quality of life | ||||
| Emotional distress | 21 (95) | 26 (87) | 18 (82) | 24 (77) |
| Body image | 12 (55) | 12 (40) | 10 (45) | 8 (26) |
| Perceived stigmatization | 10 (45) | 3 (10) | 8 (36) | 1 (3) |
| Ability to cope | 9 (41) | 2 (7) | 6 (27) | 2 (6) |
| Social relations: satisfaction with participation in social activities | 14 (64) | 9 (30) | NA | NA |
| Social relations: ability to participate in social roles and activities | 14 (64) | 4 (13) | NA | NA |
| General physical functioning | 15 (68) | 9 (30) | NA | NA |
| 2b. Quality of life of family: delivery of care | ||||
| Satisfied with treatment | 15 (68) | 17 (57) | 15 (68) | 12 (39) |
| 2c. Quality of life of family: family function | ||||
| Acceptance of parents/family members of having a child/family member with CMN | 7 (32) | 3 (10) | NA | NA |
| 3. Neoplasms (cancer) | ||||
| Presence of melanoma | 22 (100) | 29 (97) | 21 (95) | 31 (100) |
| Presence of other malignancy | 19 (86) | 16 (53) | 19 (86) | 18 (58) |
| 4. Nervous system | ||||
| Neurological symptoms and signs | 21 (95) | 28 (93) | 20 (91) | 29 (94) |
| Neuroimaging findings (documented/measured when neuroimaging is performed) | 17 (77) | 16 (53) | 18 (82) | 19 (61) |
| Brain complications due to melanosis, melanoma or metastasis | 10 (45) | 7 (23) | 8 (36) | 6 (19) |
| 5. General adverse events (problems) | ||||
| Wound problems of the CMN (after treatment and when spontaneous wounds of the CMN occur) | 20 (91) | 25 (83) | 20/21 (95) | 24 (77) |
| Scar problems | 20 (91) | 24 (80) | 20/21 (95) | 23 (74) |
| Anatomical deformation | 19 (86) | 19 (63) | 19/21 (90) | 18 (58) |
| 6. Pathology | ||||
| Histological characteristics (documented/measured when skin removal is performed) | NA | NA | 15 (68) | 20 (65) |
| Molecular characteristics (documented/measured when available) (outcome proposed during consensus meeting) | NA | NA | NA | 22 (71) |
Data are n (%). Provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders and the results of voting after the first and second voting round. Outcome included in the core domain set are presented in bold. One participant did not vote for the care setting in the second round. CMN, congenital melanocytic naevi; NA, not applicable.
Figure 1.
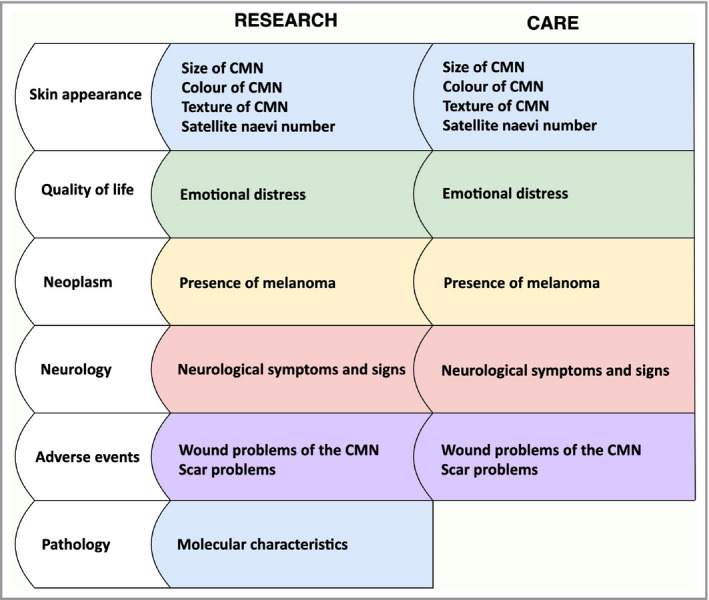
Core domains (white) and outcomes (coloured) of the core domain set of congenital melanocytic naevi (CMN) of care and research
Discussion
In this study, we reached consensus on which outcomes should describe the CDS of care and research in CMN. The provisional list of 28 possible outcomes was reordered and limited to nine core outcomes for care and 10 mostly redundant core outcomes for research. This limited number of outcomes makes the CDS feasible for use in all future care and research settings. 25 As the outcomes included in the CDS are ‘core’, they are of the highest priority for inclusion in all clinical and research outcome evaluations. Of course, other outcomes beyond those considered as core may always be measured additionally.
The size, colour and texture of the CMN, as well as a proxy for the number of ‘satellite’ (disseminated) naevi, are included in the CDS. These aspects are also recommended as baseline characteristics to be reported by a consensus‐derived, internationally used classification developed by Krengel et␣al., 9 and qualified (the ‘6B’ and ‘biker glove’ distributions) 26 , 27 for the location of the CMN. We highly recommend using these classifications in all CMN reports to obtain uniform descriptions of CMN. However, these classifications are not themselves outcome measurement instruments and are not designed to evaluate responses to interventional treatment or spontaneous changes after watchful waiting. In future research, outcome measurement instruments must be identified or developed, and validated, to measure the dynamics of size, colour, texture and number of additional naevi in order to evaluate CMN management.
We did not identify specific neurological symptoms/signs that should be measured. We believe that specific outcomes should be decided with stakeholders experienced in neurological outcomes in patients with CMN. Future research may refine this recommendation by defining how this should be measured (e.g. examination by neurologist/physician or a patient‐reported outcome measurement instrument). In this process, it should be decided which specific neurological symptoms and signs should always be assessed in care and research. To assist this process, the OCOMEN team has conducted a systematic review to identify which specific neurological symptoms/signs have been described in patients with CMN (PROSPERO ID: CRD42020177555).
Some outcomes that were highlighted as important during the consensus meeting (e.g. ‘neuroimaging findings’ were not voted for by > 70% of stakeholders). It is not feasible to measure too many outcomes, and stakeholders voted other outcomes to be of greater importance. 25 When possible, the excluded outcomes could be measured/documented alongside the core outcomes in research and care.
We used a broad network to involve patients, parents and professionals, attempting to reach and engage everybody who wanted to participate. All participants had the opportunity to state their opinions via a questionnaire, email, telephone and during the consensus meeting. All the feedback was considered to improve the list of outcomes.
A limitation was that the group of patients and parents became under‐represented in the group of participants. We tried to reach as many patients and parents as possible; however, there were more professionals willing to participate in this project. Social media posts in patient support groups were viewed by an estimated 4000 subscribers, but only four of these subscribers showed an interest in participating. We did not exclude professionals to maintain equal proportions, as we did not want to exclude anybody who was willing to participate. Most of these professionals were dermatologists and surgeons, which may have influenced the choice of outcomes. Nevertheless, a large representation of dermatologists and plastic surgeons tallies with the prominent role of these professionals in the care and research of CMN, and also reflects their voluntary participation in interdisciplinary meetings devoted to CMN. 6 , 28 Only a limited number of outcomes were included in the CDS. Many questions are still unanswered concerning outcomes for such fields as neuroimaging or psychological functioning. Standard reporting of outcomes in these different fields will greatly improve knowledge about the impact and treatment of CMN. However, when too many outcomes are recommended to be measured, the CDS may not be widely adopted, which could impede uniformity in outcome reports. 24 , 25
The next step is to define the core outcome measurement set for CMN. To reach uniformity, the core outcomes should be measured by standard outcome measurement instruments. Relevant stakeholders should try to reach consensus on which outcome measurement instruments should be used to measure the domains and outcomes included in the CDS of CMN.
Author Contribution
Anne Fledderus: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Methodology (lead); Project administration (lead); Resources (equal); Software (lead); Validation (lead); Visualization (lead); Writing‐original draft (lead). S. Pasmans: Conceptualization (equal); Data curation (supporting); Methodology (equal); Supervision (equal); Writing‐original draft (equal). Albert Wolkerstorfer: Conceptualization (equal); Data curation (supporting); Formal analysis (equal); Methodology (equal); Writing‐original draft (equal). Welling Oei: Conceptualization (equal); Data curation (supporting); Methodology (equal); Writing‐original draft (equal). Heather Etchevers: Conceptualization (equal); Data curation (supporting); Methodology (equal); Writing‐original draft (equal). Marjolein van Kessel: Conceptualization (supporting); Data curation (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Chantal van der Horst: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (lead); Writing‐original draft (equal). Phyllis Spuls: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Supervision (lead); Validation (equal); Visualization (equal); Writing‐original draft (equal).
Supporting information
Table␣S1 List 1: provisional list of outcomes of the core domain set of both care and research of congenital melanocytic naevi. Obtained in previous research. 8
Table␣S2 List of participants.
Table␣S3 List 2: provisional list of outcomes reviewed by the OCOMEN team.
Table␣S4 List 3: provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders.
Appendix␣S1 Definitions of the outcomes of the provisional list of outcomes.
Video␣S1 Author video.
Powerpoint S1 Journal Club Slide Set.
Acknowledgments
We thank the stakeholders who contributed in this project include Avani Adhia (India); Leon van Adrichem (the Netherlands); Helene Aubert (Denmark); Jean Baptiste (USA); Sebastien Barbarot (France); Benjamin Bloom (Germany); Olivia Boccara (France); Wiatt Bowers (USA); Ingeborg Cappelen Lindheim (Norway); Anne Dekerk (Belgium); Remco van Doorn (the Netherlands); Alsaiyd Eman (Canada); Marlies de Graaf (the Netherlands); Lotte Haverman (the Netherlands); Susanne von der Heydt (Germany); Gabriella Hublova (Czech Republic); Isabelle James (France); Yasmin Khakoo (USA); Oren Lapid (the Netherlands); Quenito Lilliana (Kenia); Saskia Maas (the Netherlands); Ornella Masnari (Switzerland); Evangelia Parzali (Greece); Ester Prooij (the Netherlands); Giovanni Raciti (Italy); Ngatse Mardoche Raden (Congo); Linda De Raeve (Belgium); Miguel Reyes‐Múgica (USA); Sieglinde McKeown (South Africa); Rosalba Semeraro (Italy); Alejandra Tomás (Spain); Michel Willemse (the Netherlands); and Christos Zouboulis (Germany). Furthermore, we would like to thank Jan Kottner for reviewing the study protocol; Lotte Haverman for reviewing the provisional outcomes of the ‘quality of life’ domain; and Max Lokhorst for his methodological support. This project is part of the European Reference Network SKIN thematic group ‘Cutaneous Mosaic Disorders Naevi and Naevoid skin disorders, complex vascular malformations and vascular tumors (https://ern‐skin.eu/thematic‐groups3/thematic‐group‐cutaneous‐mosaic‐disorders‐naevi‐naevoid‐skin‐disorders‐complex‐vascular‐malformations‐and‐vascular‐tumors/).
Funding sources This project was supported, in part, by a grant from the European Academy of Dermatology and Venereology to S.G.M.A.P., and by Stichting de Merel. H.C.E. was supported by funding from the Association Naevus Géant Congénital, Naevus 2000 France‐Europe and the Asociación Española de Nevus Gigante Congénito.
Conflicts of interest The authors declare that they have no conflicts of interest.
S.G.M.A.P. and A.W. share second authorship.
Plain language summary available online
References
- 1. Koot HM, de Waard‐van der Spek F , Peer CD, et␣al. Psychosocial sequelae in 29 children with giant congenital melanocytic naevi. Clin Exp Dermatol 2000; 25:589–93. [DOI] [PubMed] [Google Scholar]
- 2. Krengel S, Hauschild A, Schafer T. Melanoma risk in congenital melanocytic naevi: a systematic review. Br J Dermatol 2006; 155:1–8. [DOI] [PubMed] [Google Scholar]
- 3. Bittencourt FV, Marghoob AA, Kopf AW et␣al. Large congenital melanocytic nevi and the risk for development of malignant melanoma and neurocutaneous melanocytosis. Pediatrics 2000; 106:736–41. [DOI] [PubMed] [Google Scholar]
- 4. Eggen CAM, Lommerts JE, van Zuuren EJ et␣al. Laser treatment of congenital melanocytic naevi: a systematic review. Br J Dermatol 2018; 178:369–83. [DOI] [PubMed] [Google Scholar]
- 5. Fledderus A, Franke C, Eggen C et␣al. Outcomes and measurement instruments used in congenital melanocytic naevi research: a systematic review. J Plast Reconstr Aesthet Surg 2020; 73:703–15. [DOI] [PubMed] [Google Scholar]
- 6. Pasmans S, Eggen C, Bergman W et␣al. Multidisiplinaire richtlijn congenitale melanocytaire naevi. Available at: https://www.huidhuis.nl/sites/huidhuis.nl/files/inline‐images/PDF/Definitieve%20richtlijn%20CMN.pdf (last accessed 7 June 2021).
- 7. Williamson PR, Altman DG, Bagley H et␣al. The COMET Handbook: version 1.0. Trials 2017; 18:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oei W, Fledderus A, Spuls P et␣al. Development of an international core domain set for medium, large and giant congenital melanocytic nevi as a first step towards a core outcome set for clinical practice and research. Br J Dermatol 2020. DOI: 10.1111/bjd.19694 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 9. Krengel S, Scope A, Dusza SW et␣al. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol 2013; 68:441–51. [DOI] [PubMed] [Google Scholar]
- 10. Oei W, Fledderus AC, Korfage I et␣al. Protocol for the development of core set of domains of the core outcome set for patients with congenital melanocytic naevi (OCOMEN project). J Eur Acad Dermatol Venereol 2020; 34:267–73. [DOI] [PubMed] [Google Scholar]
- 11. Dodd S, Clarke M, Becker L et␣al. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018; 96:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kirkham JJ, Davis K. Core Outcome Set – STAndards for Development: the COS‐STAD recommendations. PLoS Medicine 2017; 14:e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitt J, Deckert S, Alam M et␣al. Report from the kick‐off meeting of the Cochrane Skin Group Core Outcome Set Initiative (CSG‐COUSIN). Br J Dermatol 2016; 174:287–95. [DOI] [PubMed] [Google Scholar]
- 14. Kirkham JJ, Gorst S, Altman DG et␣al. Core Outcome Set‐STAndards for Reporting: the COS‐STAR statement. PLoS Medicine 2016; 13:e1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmitt J, Spuls P, Boers M et␣al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67:1111–7. [DOI] [PubMed] [Google Scholar]
- 16. Chalmers JR, Thomas KS, Apfelbacher C et␣al. Report from the fifth international consensus meeting to harmonize core outcome measures for atopic eczema/dermatitis clinical trials (HOME initiative). Br J Dermatol 2018; 178:e332–e341. [DOI] [PubMed] [Google Scholar]
- 17. Fledderus AC, Pasmans SGMA, Wolkerstorfer A et␣al. Identify the outcomes describing the domains of the core outcome set of congenital melanocytic naevi: a protocol. Available at: http://cs‐cousin.org/wordpress/wp‐content/uploads/2021/03/Protocol‐OCOMEN‐outcomes‐describing‐domains.pdf (last accessed 1 June 2021).
- 18. PROMIS . List of adult measures. Available at: http://www.healthmeasures.net/explore‐measurement‐systems/promis/intro‐to‐promis/list‐of‐adult‐measures (last accessed 1 June 2021).
- 19. PROMIS . List of pediatric measures. Available at: http://www.healthmeasures.net/explore‐measurement‐systems/promis/intro‐to‐promis/list‐of‐pediatric‐measures (last accessed 1 June 2021).
- 20. Harrop E, Scott H, Sivell S et␣al. Coping and wellbeing in bereavement: two core outcomes for evaluating bereavement support in palliative care. BMC Palliative Care 2020; 19:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baltres A, Salhi A, Houlier A et␣al. Malignant melanoma with areas of rhabdomyosarcomatous differentiation arising in a giant congenital nevus with RAF1 gene fusion. Pigment Cell Melanoma Res 2019; 32:708–13. [DOI] [PubMed] [Google Scholar]
- 22. Ulrich M, Tinschert S, Siebert E et␣al. Detection of a multilineage mosaic NRAS mutation c. 181C> A (p. Gln61Lys) in an individual with a complex congenital nevus syndrome. Pigment Cell Melanoma Res 2019; 32:470–3. [DOI] [PubMed] [Google Scholar]
- 23. da Silva VM , Martinez‐Barrios E, Tell‐Martí G et␣al. Genetic abnormalities in large to giant congenital nevi: beyond NRAS mutations. J Invest Dermatol 2019; 139:900–8. [DOI] [PubMed] [Google Scholar]
- 24. Tong A, Crowe S, Gill JS et␣al. Clinicians’ and researchers’ perspectives on establishing and implementing core outcomes in haemodialysis: semistructured interview study. BMJ Open 2018; 8:e021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmitt J, Lange T, Kottner J et␣al. Cochrane reviews and dermatological trials outcome concordance: why Core Outcome Sets could make trial results more usable. J Invest Dermatol 2019; 139:1045–53. [DOI] [PubMed] [Google Scholar]
- 26. da Silva VPM , Marghoob A, Pigem R et␣al. Patterns of distribution of giant congenital melanocytic nevi (GCMN): the 6B rule. J Am Acad Dermatol 2017; 76:689–94. [DOI] [PubMed] [Google Scholar]
- 27. Kittler NW, Mathes EF, Kinsler V et␣al. The biker‐glove pattern of congenital melanocytic nevi. Pediatr Dermatol 2019; 36:918–21. [DOI] [PubMed] [Google Scholar]
- 28. Ott H, Krengel S, Beck O et␣al. Multidisciplinary long‐term care and modern surgical treatment of congenital melanocytic nevi – recommendations by the CMN surgery network. J Dtsch Dermatol Ges 2019; 17:1005–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table␣S1 List 1: provisional list of outcomes of the core domain set of both care and research of congenital melanocytic naevi. Obtained in previous research. 8
Table␣S2 List of participants.
Table␣S3 List 2: provisional list of outcomes reviewed by the OCOMEN team.
Table␣S4 List 3: provisional list of outcomes reviewed by the OCOMEN team and relevant stakeholders.
Appendix␣S1 Definitions of the outcomes of the provisional list of outcomes.
Video␣S1 Author video.
Powerpoint S1 Journal Club Slide Set.


