Abstract
In August 2017, cyclin‐dependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy have been reimbursed in the Netherlands for patients with hormone receptor positive (HR+), HER2 negative (HER2−) advanced breast cancer (ABC). This study evaluates the implementation of CDK4/6 inhibitors and changes in treatment choices in the Netherlands. All patients diagnosed with HR+/HER2− ABC in 2009 to 2018 in seven hospitals were selected from the Southeast Netherlands Advanced Breast cancer (SONABRE) registry. The 2‐year cumulative use of CDK4/6 inhibitors since reimbursement date (August 2017) was assessed using competing‐risk methodology in two cohorts. The first cohort included patients with ABC diagnosis between August 2017 and December 2018. The second cohort included patients with ABC diagnosis between 2009 and August 2017, and still alive on August 1, 2017. In addition, treatment choices in the first three lines of therapy in calendar years 2009 to 2018 were evaluated for the total study population. Among patients diagnosed since August 2017 (n = 214), 50% (95% confidence interval [CI] = 43‐57) received CDK4/6 inhibitors within 2 years beyond diagnosis. Of eligible patients diagnosed before August 2017 (n = 417), 31% (95% CI = 27‐36) received CDK4/6 inhibitors within 2 years following reimbursement. Another 20% of both cohorts are still CDK4/6 inhibitor naïve and on first‐line therapy. The use of chemotherapy decreased in first two lines of therapy between 2009 and 2018 (first‐line: 29%‐13%; second‐line: 26%‐19%). The implementation rate of CDK4/6 inhibitors since reimbursement is currently 50% within 2 years beyond diagnosis and is expected to increase further. The implementation of targeted therapy decreased the use of chemotherapy as first‐line therapy.
Keywords: breast cancer, CDK4/6 inhibitors, implementation, metastatic disease, real‐world
Short abstract
What's new?
In the Netherlands, inhibitors of cyclin‐dependent kinase 4/6 (CDK 4/6) are eligible for reimbursement by health insurers. The present report describes implementation patterns of CDK4/6 inhibitors for the treatment of advanced breast cancer since 2017, based on data and observations collected from seven hospitals across the Southeast Netherlands. Analyses show that about half of patients with HR+/HER2‐ metastatic breast cancer are treated with CDK4/6 inhibitors. Following the implementation of these therapies, use of first‐line chemotherapy decreased significantly. Reduced chemotherapy use may have beneficial effects on quality of life for patients, adding value to overall gains in survival.
Abbreviations
- ABC
advanced breast cancer
- AI
aromatase inhibitor
- CDK4/6
cyclin‐dependent kinase 4/6
- CI
confidence interval
- ET
endocrine therapy
- HER2
human epidermal growth factor receptor 2
- HR
hormone receptor
- mTOR
mechanistic target of rapamycin
1. INTRODUCTION
About two‐thirds of advanced breast cancer (ABC) diagnoses are of the hormone receptor (HR) positive, human epidermal growth factor receptor 2 (HER2) negative (HR+/HER2−) subtype. 1 For years, the best available treatment plan for patients diagnosed with HR+/HER2− ABC was using different lines of endocrine therapy (ET) until development of endocrine resistance, followed by various lines of chemotherapy as escalation strategy. 2 , 3 Currently, new targeted therapies are being designed to prolong the efficacy of ET while targeting activated cell cycle pathways. 4
Following the registration of the mechanistic target of rapamycin (mTOR) inhibitor everolimus in 2012, cyclin‐dependent kinase 4/6 (CDK4/6) inhibitors have recently been approved for HR+/HER2− ABC. CDK4/6 inhibitors halt the proliferation of breast cancer cells by arresting progression from the G1 to the S phase during cell division, as CDK4 and CDK6 are important key drivers for the stimulation of cell cycle transcription genes. 5 , 6 In 2015, palbociclib was the first CDK4/6 inhibitor to be approved by the Food and Drug Administration, as treatment for HR+/HER2− ABC in combination with aromatase inhibitor (AI) or fulvestrant as first‐ or next‐line therapy. The European Medicines Agency then approved palbociclib in November 2016 and in the Netherlands palbociclib could be prescribed in an early access program in 2016 and was reimbursed from August 2017. Shortly thereafter two more CDK4/6 inhibitors, ribociclib (May 2018) and abemaciclib (September 2019), were reimbursed as well. All three CDK4/6 inhibitors have shown a significant and clinically relevant improvement in progression‐free survival of 8.8 months over ET alone, as presented in a pooled‐analysis by Gao et al. 7 In addition, in the clinical trials all CDK4/6 inhibitors showed an overall survival benefit. 8 , 9 , 10 , 11
Recently, data from the German PRAEGNANT Registry has reported that the use of CDK4/6 inhibitors in the second year beyond reimbursement comprised 64% of first‐line therapies. 12 The proportion of patients receiving CDK4/6 inhibitors in next‐line therapy for patients who were CDK4/6 inhibitor naïve was not reported. In a budget impact analysis for the Netherlands, the total market size was expected to be approximately 80% to 90% of newly diagnosed patients with HR+/HER2− ABC as first‐ or second‐line therapy. 13 Whether CDK4/6 inhibitors plus ET is best given as first‐ or second‐line of palliative therapy is unclear and important issues to consider are disease burden, efficacy, quality of life and costs. 14 To compare these strategies directly, the SONIA study is running in the Netherlands as of November 2017. It is a multicenter randomized phase III study comparing AI plus CDK4/6 inhibition in first‐line followed by fulvestrant in second‐line to AI in first‐line followed by fulvestrant plus CDK4/6 inhibition in second‐line. 15
Gaining insights into the implementation of CDK4/6 inhibitors in real‐life is important to learn how they are used in daily clinical practice. Therefore, we present an unselected real‐world study evaluating the implementation of CDK4/6 inhibitors among all patients systemically treated for HR+/HER2− ABC before and beyond reimbursement in the Southeast of the Netherlands. Furthermore, we evaluate changes in other treatment choices in HR+/HER2− ABC related to the introduction of CDK4/6 inhibitors.
2. METHODS
2.1. Patients
Patients diagnosed with HR+/HER2− ABC in 2009 to 2018 were identified from the SOutheast Netherlands Advanced BREast cancer (SONABRE) registry, NCT‐03577197. In the SONABRE registry, all patients diagnosed with ABC and aged above 18 years were included. Specially trained registrars retrospectively collected information from medical files, including patient and tumor characteristics, treatment details (surgery, radiotherapy and [neo‐]adjuvant and palliative systemic therapy) and date and cause of death. The Medical Research Ethics Committee of Maastricht University Medical Centre approved the registry (15‐4‐239). For this present study, we selected patients who were systemically treated for HR+/HER2− ABC in seven hospitals, including one academic, three teaching and three nonteaching hospitals. Follow‐up was collected in 2020, data‐lock was on October 9, 2020.
2.2. Definitions
HR status was classified as positive if the cancer cells had an estrogen or progesterone receptor positivity of ≥10% of one or both receptors. HER2 receptor status was defined negative in case of an immunohistochemistry score of 0 or 1+ or a negative in situ hybridization result. Receptor status of the last available biopsy was used, that is, biopsy of metastasis, local recurrence or otherwise the primary tumor. Metastatic‐free interval was defined as the interval between the date of primary breast cancer diagnosis and the date of diagnosis of metastatic disease. De novo metastatic disease was defined as the diagnosis of metastatic disease within 3 months from diagnosis of the primary tumor.
2.3. Statistical analyses
The primary study goal was to evaluate the implementation of CDK4/6 inhibitors in two cohorts of HR+/HER2− ABC patients diagnosed before and beyond reimbursement date of the first CDK4/6 inhibitor in the Netherlands (August 2017). In the first cohort, patients with ABC diagnosis from August 2017 until December 2018 were included and endpoints were the 2‐year rate of CDK4/6 inhibitor use beyond date of diagnosis, and choices in first‐ and second‐line therapy. In the second cohort, patients with ABC diagnosis between 2009 and August 2017 and still alive and CDK4/6 inhibitor naïve on August 1, 2017 were included; endpoints were the 2‐year rate of CDK4/6 inhibitor use beyond reimbursement date and choice in first‐given next line of therapy since reimbursement date. Patients treated with CDK4/6 inhibitors in early access programs or clinical trials before reimbursement were thus not included in the second cohort. Contrarily, we did include patients treated in the CompLEEment‐1 (NCT‐02941926) or SONIA (NCT‐03425838) trials beyond August 2017 in the cohorts. The cumulative use of a CDK4/6 inhibitor was assessed using competing risk methodology, defining use of CDK4/6 inhibitors as “event” and death without the use of CDK4/6 inhibitors as “competing event.” Patients in follow‐up were censored at the date of last update. The number at risk consisted of patients still eligible for systemic therapy without prior prescription of a CDK4/6 inhibitor. Two‐year rates were reported, in line with the follow‐up period since August 2017.
The secondary study goal was to evaluate the treatment choices in the first three lines of therapy in all patients diagnosed with HR+/HER2− ABC per calendar year from 2009 to 2018. We estimated the proportion of patients starting a next line of therapy by using the Kaplan‐Meier methodology, using line of therapy as time variable. Chi‐square test for trend was used to study trends in treatment choices over the calendar years.
3. RESULTS
3.1. Patient characteristics
Of 1481 patients diagnosed with HR+/HER2− ABC, 1407 (95%) patients were systemically treated and included in the analyses (Figure 1). Of the patients eligible for CDK4/6 inhibitors since reimbursement date, 214 patients were diagnosed with ABC beyond, and 417 patients before the reimbursement date of CDK4/6 inhibitors. In the total group, median age was 66.1 years (range, 29‐98), 95% of patients had a WHO performance status of 0 to 2, and 57% had coexisting morbidities at ABC diagnosis (Table 1). Of all patients, 25% had de novo metastatic disease, 8% a metastatic‐free interval of 3 to 24 months and 66% a metastatic‐free interval over 24 months.
FIGURE 1.
Patient selection for the evaluation of treatment choices and the implementation of CDK4/6 inhibitors. CDK4/6, cyclin‐dependent kinase 4/6
TABLE 1.
Patient characteristics at time of advanced disease diagnosis for the total group of patients systemically treated for HR+/HER2− ABC, and for the cohorts of patients eligible for CDK4/6 inhibitors since the reimbursement date (August 2017) and diagnosed before and beyond this date
| Patient characteristics | Total group, ABC diagnosis 2009‐2018 (n = 1407) | ABC diagnosis 2009‐July 2017 (n = 417) | ABC diagnosis August 2017‐December 2018 (n = 214) |
|---|---|---|---|
| Sex, female | 1395 (99) | 413 (99) | 213 (100) |
| Age (y), median (range) | 66.1 (29‐98) | 66.2 (32‐98) | 66.7 (29‐91) |
| WHO performance status a | |||
| 0‐2 | 1051 (95) | 339 (97) | 183 (92) |
| 3‐4 | 56 (5) | 9 (3) | 16 (8) |
| Unknown | 300 | 66 | 12 |
| Comorbidities | |||
| Any | 806 (57) | 235 (56) | 125 (58) |
| Pulmonary | 137 (10) | 38 (9) | 29 (14) |
| Cardiovascular | 547 (39) | 154 (37) | 81 (38) |
| Cerebrovascular | 105 (8) | 33 (8) | 21 (10) |
| Other malignancy | 119 (9) | 34 (8) | 17 (8) |
| Histology | |||
| Ductal carcinoma | 1012 (75) | 301 (75) | 157 (76) |
| Lobular carcinoma | 318 (23) | 93 (23) | 45 (22) |
| Other | 27 (2) | 10 (2) | 5 (2) |
| Unknown | 50 | 13 | 7 |
| Hormone receptor status | |||
| ER positive | 1402 (100) | 417 (100) | 214 (100) |
| PR positive | 870 (62) | 282 (68) | 125 (58) |
| Initial metastatic sites | |||
| Nonvisceral | 1215 (86) | 365 (88) | 185 (86) |
| Bone only | 457 (33) | 157 (38) | 72 (34) |
| Soft tissue | 481 (34) | 150 (36) | 71 (33) |
| Visceral b | 739 (53) | 174 (42) | 110 (51) |
| Lung | 297 (21) | 74 (18) | 36 (17) |
| Liver | 362 (26) | 67 (16) | 58 (27) |
| Pleura | 210 (15) | 56 (13) | 41 (19) |
| Central nervous system | 52 (4) | 13 (3) | 5 (2) |
| Initial number of metastatic sites | |||
| 1 | 652 (46) | 221 (53) | 104 (48) |
| 2 | 410 (29) | 113 (27) | 68 (32) |
| ≥3 | 345 (25) | 83 (20) | 42 (20) |
| Metastatic‐free interval c | |||
| De novo (<3 mo) | 355 (25) | 116 (28) | 51 (24) |
| 3‐23 mo | 118 (8) | 22 (5) | 18 (8) |
| ≥24 mo | 934 (66) | 279 (67) | 145 (68) |
| Prior (neo‐)adjuvant therapy d | |||
| Endocrine therapy | 741 (70) | 184 (61) | 79 (48) |
| Chemotherapy | 502 (47) | 127 (42) | 117 (72) |
Note: Data given as number (%) unless otherwise indicated.
Abbreviations: ABC, advanced breast cancer; CDK4/6, cyclin‐dependent kinase 4/6; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; PR, progesterone receptor; WHO, World Health Organization.
WHO performance status is measured on a 5‐point scale, WHO 0 indicates no restrictions in activity and higher numbers indicate increasing inability.
Visceral metastases: liver, lung, pleura, peritoneal and/or gastrointestinal metastases.
Metastatic‐free interval is defined as interval between date of breast cancer and date of ABC diagnosis.
Among patients with recurrent metastases (excluding patients with de novo ABC).
3.2. Patients with ABC diagnosis beyond reimbursement
Among the 214 patients with ABC diagnosis beyond August 2017, median follow‐up time was 25 months (interquartile range, 21‐29). The cumulative use of CDK4/6 inhibitors at 2 years beyond ABC diagnosis was 50% (95% confidence interval [CI] = 43‐57) (Figure 2A). Another 17% (95% CI = 12‐23) of patients had died without CDK4/6 inhibitor use at 2 years beyond diagnosis (Figure S1). At last follow‐up, 69 patients (32%) were alive and did not receive treatment with CDK4/6 inhibitors (yet), including 47 patients (22%) still receiving first‐line therapy.
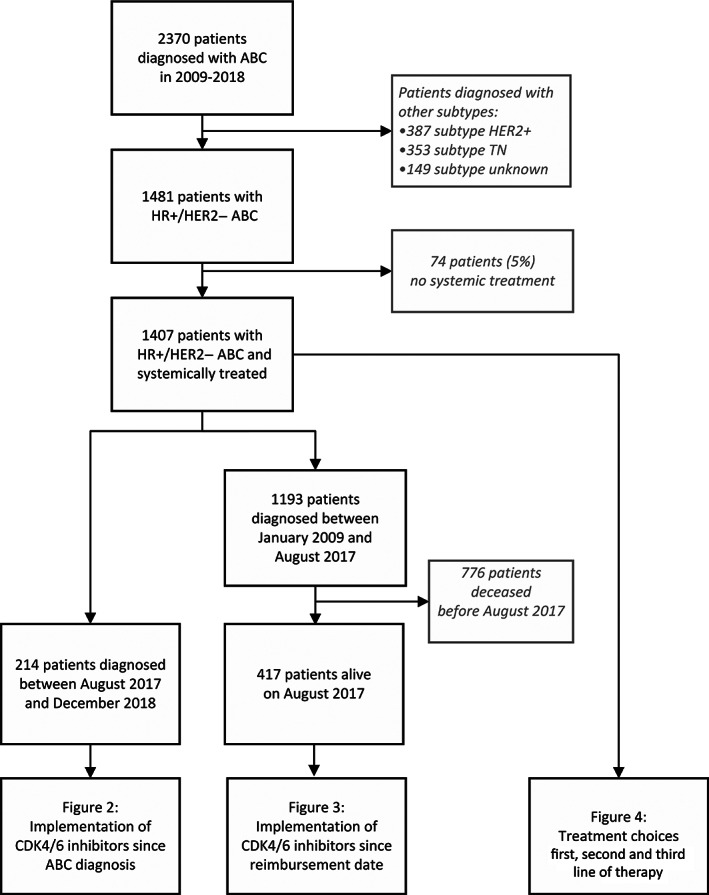
FIGURE 2.
The use of CDK4/6 inhibitors in patients diagnosed with HR+/HER2− ABC since August 2017. (A) Cumulative use of CDK4/6 inhibitors since date of ABC diagnosis by competing risk methodology. (B) First‐line treatment choice for all patients (n = 214), and second‐line treatment choice for CDK4/6 inhibitor naïve patients (n = 71). ABC, advanced breast cancer; CDK4/6, cyclin‐dependent kinase 4/6; CDK4/6i, CDK4/6 inhibitors; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor [Color figure can be viewed at wileyonlinelibrary.com]
In first‐line therapy, 31% of patients received a CDK4/6 inhibitor, 57% ET and 12% chemotherapy (Figure 2B). Of CDK4/6 inhibitor naïve patients who started second‐line therapy during follow‐up (n = 71), 44% received a CDK4/6 inhibitor, 38% ET and 18% chemotherapy. Among this cohort, 16 patients (7%) were enrolled in the SONIA study.
3.3. Patients with ABC diagnosis before reimbursement
In 417 eligible patients with ABC diagnosis between 2009 and August 2017, the number of prior lines of palliative therapy was 1 (range, 0‐10), and of them 18% had a previous line with chemotherapy. At 2 years beyond reimbursement date, the cumulative use of CDK4/6 inhibitors in this cohort was 31% (95% CI = 27‐36) (Figure 3A). Another 27% (95% CI = 23‐32) died without CDK4/6 inhibitor use (Figure S2). At last follow‐up, 105 patients (25%) were alive and did not switch systemic therapy since August 2017, including 72 patients (17%) receiving first‐line therapy.
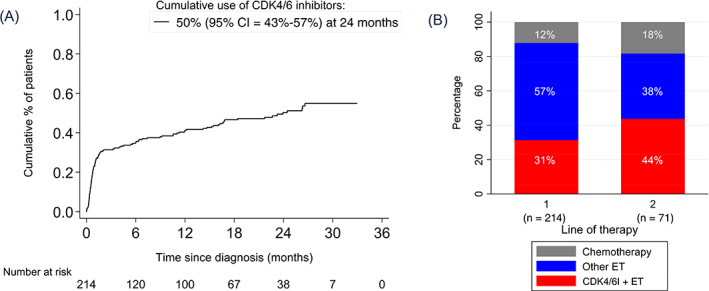
FIGURE 3.
The use of CDK4/6 inhibitors since the reimbursement date in patients diagnosed with HR+/HER2− ABC between 2009 and August 2017, and alive on August 1, 2017. (A) Cumulative use of CDK4/6 inhibitors since reimbursement date by competing risk methodology. (B) First‐given new line of therapy since reimbursement date in patients that switched systemic therapy (n = 248). ABC, advanced breast cancer; CDK4/6, cyclin‐dependent kinase 4/6; CDK4/6i, CDK4/6 inhibitors; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; mTOR, mTOR inhibitors [Color figure can be viewed at wileyonlinelibrary.com]
In 248 patients (59%), a new line of therapy was started between August 2017 and the end of follow‐up. As first‐given new line of therapy since reimbursement date, CDK4/6 inhibitors were started in 46% of patients, ET in 36%, mTOR inhibitor in 3% and chemotherapy in 15% (Figure 3B). Findings were not dependent on period of ABC diagnosis (data not further shown).
3.4. Treatment choices for first three lines of therapy in all patients
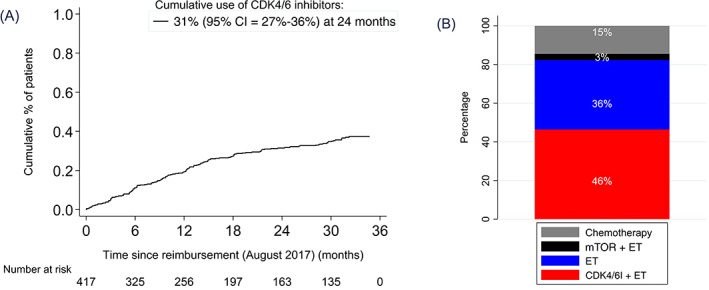
In the total group of systemically treated HR+/HER2− ABC patients, 83% received a second‐line therapy and 67% a third‐line. The use of chemotherapy as first‐line therapy decreased from 29% (95% CI = 21‐38) in 2009 to 13% (95% CI = 8‐19) in 2018 (P for trend <.001) and as second‐line therapy from 26% (95% CI = 16‐39) in 2010 to 19% (95% CI = 12‐28) in 2018 (P for trend = .07) (Figure 4). The use of endocrine monotherapy decreased in all three lines of therapy (P for trend ≤.001). Regarding mTOR inhibitor use in second‐line, we observed the highest uptake in 2014 (28%), afterward gradually decreasing over time. For 2018, the first year CDK4/6 inhibitors were widely available, their prescriptions covered 33% of all first‐line therapies, 48% of all second‐line therapies and 16% of all third‐line therapies.
FIGURE 4.
Treatment choices in all patients diagnosed with HR+/HER2− ABC from 2009 until 2018 per calendar year in first, second and third line of therapy. Treatment choices are shown from 2010 in second‐line and from 2011 in third‐line to present a representative distribution of patients in next‐line treatment. ABC, advanced breast cancer; HER2, human epidermal growth factor receptor 2; HR, hormone receptor [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
This study has presented real‐world data on the implementation of CDK4/6 inhibitors in patients diagnosed with HR+/HER2− ABC in the Southeast of the Netherlands. In patients diagnosed since the reimbursement of CDK4/6 inhibitors, the implementation rate was 50% within 2 years beyond ABC diagnosis, and 31% of first‐line therapies. Of patients diagnosed before reimbursement and still able to receive a CDK4/6 inhibitor, about 30% received it in 2 years following reimbursement. With the implementation of targeted therapy, we observed a clinically relevant reduction in the use of chemotherapy in the initial two lines of therapy.
The implementation rate in patients with ABC diagnosis since reimbursement as presented here is now 50% within the first 2 years beyond diagnosis, but might increase when the quarter of patients still on first‐line therapy will start their second‐line. In eligible patients who did proceed to next line, we observed that 45% received CDK4/6 inhibitors. Also relevant, the budget impact analysis for the Netherlands did not take into account the use of CDK4/6 inhibitors in patients diagnosed before reimbursement, while in absolute numbers this represents about half of the patients who started with CDK4/6 inhibitors therapy in this real‐world study. 13 Nevertheless, this is a phenomenon for the transitional period of the first years following on reimbursement. It is expected that eventually CDK4/6 inhibitors will be mainly used as first‐ and second‐line therapy. 16 Longer follow‐up time is needed to find out the eventual market size of CDK4/6 inhibitors. We expect that anticipated market size of 80% to 90% in the Netherlands is not realistic. The National Institute for Health and Care Excellence in the United Kingdom estimated a market share for CDK4/6 inhibitors of 60% of HR+/HER2− ABC patients, which is more realistic to achieve at current pace. 17
It is perhaps unexpected that 57% of first‐line therapies consist of endocrine monotherapy in patients diagnosed beyond reimbursement against 31% being CDK4/6 inhibitors. Contrary to international guidelines, where CDK4/6 inhibitors are advised in first or second line of therapy, it is important to note that the Dutch Society of Medical Oncology had recommended CDK4/6 inhibitors for second‐line in patients with low‐aggressive HR+/HER2− ABC, while awaiting the results of the Dutch SONIA trial on the preferred position of CDK4/6 inhibitor use. 18 , 19 Although only 7% of patients participated in the SONIA trial, we think that the statement of the Dutch Society of Medical Oncology advising CDK4/6 inhibitors in second‐line is reflected in the results of this real‐world study with a low CDK4/6 prescription rate in first‐line. Other possible reasons for low uptake in first‐line could be the toxicity profile and monitoring requirements in patients with comorbidities, higher age or low‐burden disease, or a slow market penetration. 20 Market penetration, that is, the actual use of the therapy as a rate of patient access, depends on several factors, such as budget allocations, integration in health care guidelines and prescriber awareness. In Europe, market access of new available therapies varies greatly between countries. 21 However, as an estimated total of 60% may now receive CDK4/6 inhibitors as either first‐ or second‐line therapy, while about one in six patients never reach a second‐line and one in three never reach a third‐line therapy, the delay of CDK4/6 inhibitors to later lines might be regretted.
In our cohort, we observed a statistically significant declined use of chemotherapy as first‐line therapy with the implementation of targeted therapies. The use of chemotherapy in first‐line decreased from 29% in 2009 to 13% in 2018. In the French Epidemiological Strategy and Medical Economics (ESME) cohort, first‐line palliative therapy in the period 2008 until 2014 was evaluated for AI‐sensitive patients, of them 44% received ET and 56% chemotherapy. 22 After chemotherapy induction, 59% were treated with endocrine maintenance therapy, also showing that the disease was considered as endocrine‐sensitive. In the German PRAEGNANT Registry, the use of chemotherapy in first‐line used to be 40% before the registration of CDK4/6 inhibitors, and decreased to 25% in the second year beyond registration. 12 The use of chemotherapy differs thus between countries and might lead to a different implementation pattern of CDK4/6 inhibitors. The delay of chemotherapy is considered a relevant aim for HR+/HER2− ABC patients, as it may generally be positive for a patients' quality of life. The greatest value of CDK4/6 inhibitors may therefore be the reduced use of chemotherapy in ABC patients in addition to the prolonged overall survival.
The use of real‐world data is a strength of our study, as it is of great value to facilitate insights into implementation in daily clinical practice. We have evaluated the implementation rates in patients diagnosed with advanced disease both before and beyond registration of CDK4/6 inhibitors, emphasizing physicians' treatment strategies in both groups. We specifically chose to assess the implementation rates with a competing risk methodology with death as competing risk to prevent an overestimation of the use of CDK4/6 inhibitors as opposed to a Kaplan‐Meier estimate. Our findings can be used to estimate the market size of future new therapies. Similarly, our results provide useful feedback to physicians to reflect on their treatment strategies. However, our study has certain limitations, inherent to its observational design, which may have interfered with the quality of the data generated. Furthermore, the regional study cohort may be a limitation for the generalizability of the study, since it is unknown how it translates nationally and to other countries. Notwithstanding, for collecting data on implementation, our registry is highly useful and currently the first thoroughly reporting the implementation rates. Longer follow‐up is needed to evaluate the implementation of CDK4/6 inhibitors during the first years of advanced disease. The use of CDK4/6 inhibitors is expected to grow by increasing market penetration.
5. CONCLUSION
Two years after the implementation of CDK4/6 inhibitors for HR+/HER2− disease, 50% of newly diagnosed patients received CDK4/6 inhibitors. The implementation rate is expected to further increase. Since 2009, we observe a decreased use of chemotherapy as first‐line therapy.
CONFLICT OF INTEREST
Marissa Meegdes, Sandra M. E. Geurts, Nathalie J. A. Teeuwen, Maaike de Boer and Vivianne C. G. Tjan‐Heijnen have received funding from the Netherlands Organization for Health Research and Development (ZonMw: 80‐82500‐98‐8003), Novartis BV, Roche, Pfizer, and Eli Lilly. All remaining authors have declared no conflicts of interest.
ETHICS STATEMENT
The SONABRE Registry (NCT‐03577197) was approved and the need for informed consent waived by the Medical Research Ethics Committee of Maastricht University Medical Center (METC 15‐4‐239).
Supporting information
Figure S1 Cumulative rate of patients with ABC diagnosis from August 2017 that deceased without receiving CDK4/6 inhibitors
Figure S2. Cumulative rate of patients diagnosed with ABC between 2009 and August 2017 and alive on August first that deceased without receiving CDK4/6 inhibitors
ACKNOWLEDGMENT
We thank our SONABRE registrars of the Department of Medical Oncology of Maastricht University Medical Center (MUMC+), Maastricht, the Netherlands.
Meegdes M, Geurts SME, Erdkamp FLG, et al. The implementation of CDK 4/6 inhibitors and its impact on treatment choices in HR+/HER2− advanced breast cancer patients: A study of the Dutch SONABRE Registry. Int. J. Cancer. 2022;150(1):124‐131. doi: 10.1002/ijc.33785
Funding information Novartis BV; Netherlands Organization for Health Research and Development (ZonMw), Grant/Award Number: 80‐82500‐98‐8003; Eli Lilly and Company; Pfizer; Roche
Contributor Information
Marissa Meegdes, Email: marissa.meegdes@mumc.nl.
Vivianne C. G. Tjan‐Heijnen, Email: vcg.tjan.heijnen@mumc.nl.
DATA AVAILABILITY STATEMENT
The SONABRE Registry data sets are available from the corresponding author (Vivianne C. G. Tjan‐Heijnen) upon reasonable request and through collaborative investigations.
REFERENCES
- 1. Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60‐70. [DOI] [PubMed] [Google Scholar]
- 2. Cardoso F, Senkus E, Costa A, et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307‐3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nur Husna SM, Tan HT, Mohamud R, Dyhl‐Polk A, Wong KK. Inhibitors targeting CDK4/6, PARP and PI3K in breast cancer: a review. Ther Adv Med Oncol. 2018;10:1758835918808509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choo JR, Lee SC. CDK4‐6 inhibitors in breast cancer: current status and future development. Expert Opin Drug Metab Toxicol. 2018;14(11):1123‐1138. [DOI] [PubMed] [Google Scholar]
- 6. Nebenfuehr S, Kollmann K, Sexl V. The role of CDK6 in cancer. Int J Cancer. 2020;147(11):2988‐2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gao JJ, Cheng J, Bloomquist E, et al. CDK4/6 inhibitor treatment for patients with hormone receptor‐positive, HER2‐negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2020;21(2):250‐260. [DOI] [PubMed] [Google Scholar]
- 8. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926‐1936. [DOI] [PubMed] [Google Scholar]
- 9. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2019;382(6):514‐524. [DOI] [PubMed] [Google Scholar]
- 10. Sledge GW Jr, Toi M, Neven P, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy‐MONARCH 2: a randomized clinical trial. JAMA Oncol. 2019;6(1):116‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cristofanilli M, Rugo HS, Im S‐A, et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): updated analyses from PALOMA‐3. J Clin Oncol. 2021;39(15_suppl):1000. [Google Scholar]
- 12. Schneeweiss A, Ettl J, Lüftner D, et al. Initial experience with CDK4/6 inhibitor‐based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer — data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast. 2020;54:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sweegers C. Budget impact analyse van palbociclib (Ibrance®) voor de behandeling van hormoonreceptor‐positieve, HER2‐negatieve gevorderde of gemetastaseerde borstkanker. Zorginstituut Nederland; maart 3, 2017.
- 14. Awada A, Gligorov J, Jerusalem G, Preusser M, Singer C, Zielinski C. CDK4/6 inhibition in low burden and extensive metastatic breast cancer: summary of an ESMO Open‐Cancer Horizons pro and con discussion. ESMO Open. 2019;4(6):e000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Ommen‐Nijhof A, Konings IR, van Zeijl CJJ, et al. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor‐positive advanced breast cancer ‐ the SONIA study: study protocol for a randomized controlled trial. BMC Cancer. 2018;18(1):1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maurer C, Ferreira AR, Martel S, et al. Endocrine therapy and palbociclib within a compassionate use program in heavily pretreated hormone receptor‐positive, HER2‐negative metastatic breast cancer. Breast. 2018;39:14‐18. [DOI] [PubMed] [Google Scholar]
- 17. Resource Impact Report: Abemaciclib with an Aromatase Inhibitor for Previously Untreated, Hormone Receptorpositive, HER2‐Negative, Locally Advanced or Metastatic Breast Cancer (TA563). NICE; 2019.
- 18. Honkoop AH, Bloemendal HJ. Plaatsbepaling NABON en NVMO: toepassing van palbociclib bij mammacarcinoom. Medische Oncol. 2017;20(1):27. [Google Scholar]
- 19. Cardoso F, Paluch‐Shimon S, Senkus E, et al. 5th ESO‐ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31(12):1623‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abraham J, Coleman R, Elias A, et al. Use of cyclin‐dependent kinase (CDK) 4/6 inhibitors for hormone receptor‐positive, human epidermal growth factor receptor 2‐negative, metastatic breast cancer: a roundtable discussion by The Breast Cancer Therapy Expert Group (BCTEG). Breast Cancer Res Treat. 2018;171(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 21. Jansen C, Amesz B. Improving Time to Patients Access to Innovative Oncology Therapies in Europe 2020. Vintura. Report was commissioned by EFPIA.
- 22. Jacquet E, Lardy‐Cléaud A, Pistilli B, et al. Endocrine therapy or chemotherapy as first‐line therapy in hormone receptor–positive HER2‐negative metastatic breast cancer patients. Eur J Cancer. 2018;95:93‐101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Cumulative rate of patients with ABC diagnosis from August 2017 that deceased without receiving CDK4/6 inhibitors
Figure S2. Cumulative rate of patients diagnosed with ABC between 2009 and August 2017 and alive on August first that deceased without receiving CDK4/6 inhibitors
Data Availability Statement
The SONABRE Registry data sets are available from the corresponding author (Vivianne C. G. Tjan‐Heijnen) upon reasonable request and through collaborative investigations.


