FIGURE 3.
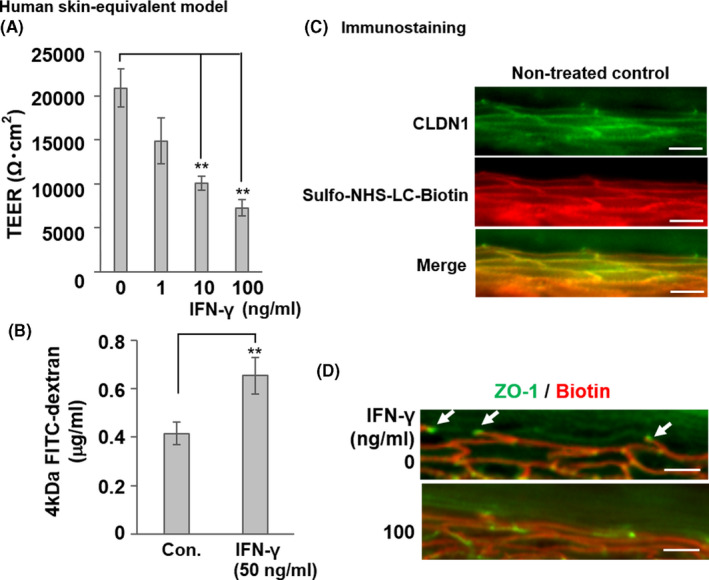
Impairment of TJ barrier by IFN‐γ in a human skin equivalent model. (a) Dose‐dependent suppression of TEER by IFN‐γ in the human skin equivalent model incubated with various IFN‐γ concentrations (1‒100 ng ml−1) for 48 h. Data are presented as the mean ± SE (n = 4). **p < 0.01 vs. control (0 ng ml−1). (B) Increased FITC‐dextran flux in the human skin equivalent model treated with 50 ng ml−1 IFN‐γ for 3 days (56.5% inhibition of TEER vs. control, *p < 0.05). Last 1 h, FITC‐dextran was added to the apical side of the skin equivalent model, and the flux to the basolateral side was measured. Data are presented as the mean ± SE (n = 4). **p < 0.01 vs. control (0 ng ml−1). (C) The normal human skin model was incubated with sulfo‐NHS‐LC‐biotin from the dermal side. After 30 min, the skin model was removed and frozen. Frozen sections were subjected to immunostaining with sulfo‐NHS‐LC‐biotin (red) to show TJ permeability and stained CLDN1 protein (green). The merged red and green image is shown in yellow. (D) Human skin equivalent models were incubated with or without IFN‐γ (100 ng ml−1) for 48 h and subjected to the TJ permeability assay with sulfo‐NHS‐LC‐biotin (red). Localization of TJs was detected using anti‐ZO‐1 (green), which was highly concentrated at the TJs (white arrows) in the skin equivalent models. Scale bars = 40 μm
