Abstract
This study was presented to observe the therapeutic effects of azathioprine (AZA) pretreatment on myocardial ischaemia reperfusion (I/R) damage in diabetic rats. All rats were randomly separated into control + sham operation; control +I/R; diabetes mellitus (DM) +I/R and DM +I/R + AZA groups. Diabetic rat models were established by intraperitoneally injecting 60 mg/kg streptozotocin (STZ). Diabetic rats were given 3 mg/kg AZA daily by gavage for 5 days. Then, myocardial I/R rat models were constructed. Myocardial infarction size and myocardial damage were respectively detected by TTC and H&E staining. Cardiac injury markers (CK‐MB and MPO) and oxidative stress factors (SOD and MDA) were measured via ELISA. The protein expression of apoptotic markers (Caspase8, Caspase3, BAX and Bcl2), inflammatory factors (TLR4 and TNF‐α) and AKT1/GSK3β in myocardial tissues was measured by western blot, immunohistochemistry or immunofluorescence. Data showed that AZA pretreatment could lessen myocardial infarction size and myocardial damage, and could down‐regulate serum CK‐MB, MPO, SOD and MDA levels in diabetic rats under I/R. Furthermore, AZA pretreatment decreased Caspase8, Caspase3, BAX, TLR4 and TNF‐α expression, and increased Bcl2 expression in myocardial tissues of diabetic rats following I/R. Also, AZA pretreatment lowered AKT1, p‐AKT1, GSK3β and p‐GSK3β expression in diabetic heart after I/R. This study found that AZA may reduce myocardial injury in diabetic rats following I/R via reducing oxidative stress, cardiomyocyte apoptosis, and inflammatory response, which could be related to AKT1/GSK3β pathway inactivation.
Keywords: apoptosis, azathioprine, diabetes, inflammation, myocardial ischaemia reperfusion, oxidative stress
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AZA
azathioprine
- CK‐MB
creatine kinase isoenzyme
- DM
diabetes mellitus
- ECG
electrocardiogram
- ELISA
enzyme‐linked immunosorbent assay
- I/R
ischaemia reperfusion
- MDA
malonaldehyde
- MPO
myeloperoxidase
- SOD
superoxide dismutase
- TTC
2,3,5‐Triphenyltetrazolium chloride
1. INTRODUCTION
Diabetes is a disorder of glucose and lipid metabolism, characterized by a marked increase in blood sugar and blood lipids, 1 which may induce perioperative complications as well as cardiovascular diseases. 2 Populations with diabetes are more subjected to be affected by cardiovascular diseases. Moreover, the cardiovascular mortality rate of diabetic patients is two to three times that of non‐diabetic subjects. 3 The diabetic heart with abnormal structure and function is easily affected by myocardial I/R injury. 4 In myocardial I/R damage, the ischaemic heart is damaged with irreversibility despite the recovery of blood flow reperfusion. Such damage often causes further necrosis and apoptosis of cardiomyocytes, mitochondrial dysfunction of viable cardiomyocytes, activation of protein kinases, and inflammatory responses, which in turn leads to poor long‐term prognosis and even death. 5 Following myocardial I/R, diabetic patients present more unfavourable clinical outcomes compared to those without diabetes. 6 Diabetes seriously destroys cardiac energy homeostasis, contributing to the disorders of cardiac function. Diabetic I/R damage has complicated, multifactorial and greatly comprehensive pathogenesis. 7 , 8 There is evidence that the diabetic heart shows resistance to therapeutic strategies for cardioprotection. 9 Thus, diabetes aggravates myocardial I/R damage. Nevertheless, effective therapeutic strategies targeting I/R damage are still lacking for diabetic subjects. 10 It is of clinical significance to understand the mechanisms against conditioning‐related cardioprotection for lessening myocardial I/R damage.
Azathioprine (AZA), a non‐specific immunosuppressant, exerts immunosuppressive effects by inhibiting the proliferation of lymphocytes in the body, 11 which is widely used in immunosuppressive therapy after transplantation and various immune diseases such as systemic lupus erythematosus, 12 inflammatory bowel disease 13 and rheumatoid arthritis. 14 Herein, this study aimed to observe the therapeutic effects of AZA pretreatment on myocardial damage, anti‐oxidative stress, anti‐apoptosis, and anti‐inflammation in diabetic rats under I/R and to explore the underlying mechanisms.
2. RESULTS
2.1. General characteristics
We measured the body weight and blood glucose before and after the establishment of the DM model, 5 days after administration of AZA and after the establishment of the I/R model. No significant difference in body weight was found between the sham, control +I/R, DM +I/R and DM +I/R + AZA groups (Figure 1A). In Figure 1B, AZA pretreatment significantly lowered the blood glucose of diabetic rats following I/R. No neurological damage occurred in mice from each group. Serum ALT and AST levels were determined after establishing the I/R model. Compared to the control +I/R group, serum ALT levels were distinctly elevated in the DM +I/R group, indicating that diabetic rats were more greatly subjected to I/R injury (Figure 1C). There was no significant difference in serum AST levels between the four groups (Figure 1D). Pretreatment with 3 mg/kg AZA did not change serum ALT and AST levels, suggesting that AZA did not damage the liver function (Figure 1C,D).
FIGURE 1.
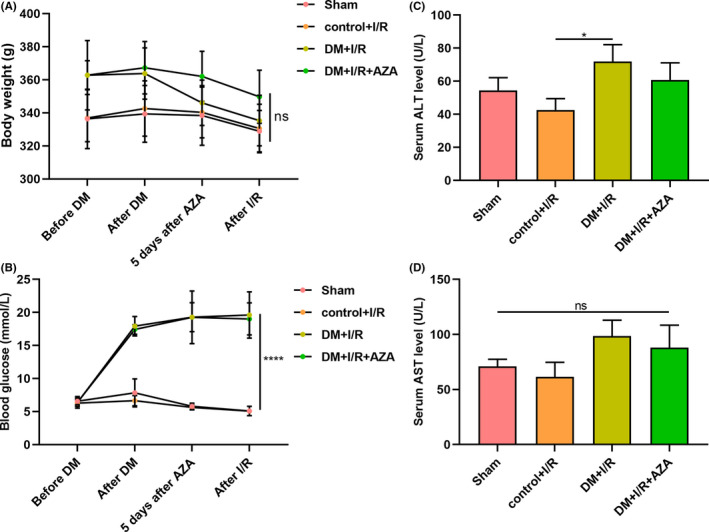
General characteristics. (A) Measurement of body weight and (B) blood glucose before and after the establishment of the DM model, 5 days after administration of AZA and after the establishment of the I/R model. (C) Determination of serum ALT and (D) AST levels in the sham, control +I/R, DM +I/R and DM +I/R + AZA groups. *p < 0.05; ****p < 0.0001 and ns: not significant. ALT, alanine aminotransferase; AZA, azathioprine; DM, diabetes mellitus; I/R, ischaemia reperfusion
2.2. AZA pretreatment improves ST segment downward shift and T wave inversion in diabetic rats following I/R
Herein, we investigated the ECG changes in four groups. The ECG of the rats in the sham group (Figure 2A) and the control +I/R group (Figure 2B) showed mild abnormalities, mild ST segment downward shift and T wave inversion. In the DM +I/R group, it was found that typical QRS complex disappeared; the towering R wave became an rsR′ complex, accompanied by a significant downward shift of the ST segment and a wide inverted T wave (Figure 2C). Compared with the DM +I/R group, the ST segment downward shift and T wave inversion were significantly improved in the DM +I/R + AZA group (Figure 2D).
FIGURE 2.

The effects of AZA pretreatment on ECG changes for diabetic rats following I/R. (A) Sham group; (B) control +I/R group; (C) DM +I/R group; (D) DM +I/R + AZA group. AZA, azathioprine; DM, diabetes mellitus; ECG, electrocardiogram; I/R, ischaemia reperfusion
2.3. AZA pretreatment lessens myocardial infarction size and myocardial injury in diabetic rats following I/R
Myocardial infarction size was measured via TTC staining (Figure 3A,B and Table 1). Compared to the sham group, myocardial infarction size was significantly higher in the control +I/R group. The rats in the DM +I/R group exhibited distinctly elevated myocardial infarction size than the control +I/R group, indicating that diabetic rats were more vulnerable to I/R. But AZA pretreatment markedly lessened myocardial infarction size of diabetic rats following I/R. As shown in the H&E staining results, compared to the control +I/R group, there was a large amount of inflammatory cell infiltration and necrosis of cardiomyocytes in the DM +I/R group (Figure 3C). However, AZA pretreatment significantly improved myocardial injury in diabetic rats after I/R.
FIGURE 3.

The effects of AZA pretreatment on myocardial infarction size and myocardial injury in diabetic rats following I/R. (A) Representative images of heart sections by TTC staining. Bar = 2 mm. (B) Quantitative results of infarct size. (C) H&E staining of heart sections. Bar = 20 μm. ****p < 0.0001. AZA, azathioprine; I/R, ischaemia reperfusion; TTC, 2,3,5‐Triphenyltetrazolium chloride
TABLE 1.
Myocardial infarct size
| Groups | n | Myocardial infarction size (mm3) |
|---|---|---|
| Sham | 6 | 0.06 |
| Control + I/R | 6 | 14.7 |
| DM + I/R | 6 | 29.8*** |
| DM + I/R + AZA | 6 | 13.2### |
| F | 72.451 | |
| P | <0.001 |
Compared to the control +I/R group. ***p < 0.001; ### p < 0.001.
2.4. AZA pretreatment lowers serum CK‐MB, MDA, MPO and SOD levels in diabetic rats following I/R
We determined serum levels of acute myocardial injury markers and oxidative stress related factors in each group. Our data showed that there were no significant changes in serum CK‐MB, MDA, MPO and SOD levels between the sham group and the control +I/R group (Figure 4A‐D). In comparison to the control +I/R group, serum CK‐MB, MDA, MPO and SOD levels were distinctly elevated in the DM +I/R group, indicating that diabetic rats were subjected to myocardial injury induced by I/R (Figure 4A‐D). But their levels were markedly lowered in the DM +I/R + AZA group than the DM +I/R group, demonstrating that AZA pretreatment could ameliorate myocardial oxidative stress in diabetic rats following I/R.
FIGURE 4.

The effects of AZA pretreatment on serum CK‐MB, MDA, MPO and SOD levels in diabetic rats following I/R. Assessment of (A) serum CK‐MB, (B) MDA, (C) MPO and (D) SOD levels by ELISA. *p < 0.05; **p < 0.01 and ns: not significant. AZA, azathioprine; CK‐MB, creatine kinase isoenzyme; I/R, ischaemia reperfusion; MDA, malonaldehyde; MPO, myeloperoxidase; SOD, superoxide dismutase
2.5. AZA pretreatment decreases apoptosis and inflammation in diabetic heart under I/R
Whether AZA pretreatment affected apoptosis and inflammation in diabetic heart under I/R was further observed by western blot (Figure 5A). The data showed that the expression of Caspase8 (Figure 5B), Caspase3 (Figure 5C) and BAX (Figure 5D) proteins was significantly increased as well as Bcl‐2 expression (Figure 5E) and Bcl‐2/Bax ratio (Figure 5F) were distinctly lessened in myocardial tissues of the DM +I/R group than the control +I/R group, suggesting that cardiomyocyte apoptosis was more likely to occur in diabetic heart under I/R. But Caspase8 (Figure 5B), Caspase3 (Figure 5C) and BAX (Figure 5D) all exhibited markedly lowered expression as well as Bcl‐2 expression (Figure 5E) and Bcl‐2/Bax ratio (Figure 5F) showed higher levels in the DM +I/R + AZA group than the DM +I/R group. These findings indicated that AZA pretreatment could lessen cardiomyocyte apoptosis in diabetic rats under I/R. Moreover, inflammation‐related proteins TLR4 and TNF‐α were measured in myocardial tissues. Higher TLR4 (Figure 5G) and TNF‐α (Figure 5H) levels were found in myocardial tissues of diabetic rats under I/R than non‐diabetic subjects following I/R. AZA pretreatment lowered their levels in diabetic myocardial tissues after I/R. To confirm the effects of AZA pretreatment on apoptosis and inflammation in diabetic heart under I/R, immunohistochemistry was presented (Figure 6A). Consistent with western blot, following I/R operation, Caspase3 (Figure 6B), Caspase8 (Figure 6C) and BAX (Figure 6D) proteins presented significantly elevated levels and Bcl‐2 (Figure 6E) showed decreased levels in myocardial tissues of diabetic rats than non‐diabetic subjects. AZA pretreatment lessened Caspase3, Caspase8 and BAX expression and increased Bcl‐2 levels in diabetic heart under I/R. Furthermore, after I/R, TLR4 (Figure 6F) and TNF‐α (Figure 6G) proteins had markedly higher expression in myocardial tissues of diabetic rats than non‐diabetic subjects, which were lowered by AZA pretreatment. The above data demonstrated that AZA pretreatment may decrease apoptosis and inflammation in diabetic heart under I/R.
FIGURE 5.

Western blot for the effects of AZA pretreatment on apoptosis and inflammation in diabetic heart under I/R. (A) Representative images. Determination of (B) Caspase8, (C) Caspase3, (D) BAX, (E) Bcl‐2, (F) Bcl‐2/Bax ratio, (G) TLR4 and (H) TNF‐α expression in heart tissues of the sham, control +I/R, DM +I/R and DM +I/R + AZA groups. ***p < 0.001 and ****p < 0.0001. AZA, azathioprine; DM, diabetes mellitus; I/R, ischaemia reperfusion
FIGURE 6.

Immunohistochemistry for the effects of AZA pretreatment on apoptosis and inflammation in diabetic rats following I/R. (A) Representative images of immunohistochemistry. Bar = 20 μm. Assessment of (B) Caspase3, (C) Caspase8, (D) BAX, (E) Bcl‐2, (F) TLR4 and (G) TNF‐α expression in heart tissues of the sham, control +I/R, DM +I/R and DM +I/R + AZA groups. **p < 0.01; ***p < 0.001 and ****p < 0.0001. AZA, azathioprine; DM, diabetes mellitus; I/R, ischaemia reperfusion
2.6. AZA pretreatment decreases cardiac AKT1/GSK3β in diabetic rats under I/R
It has been confirmed that AKT1/GSK3β is activated in myocardial I/R damage. 15 Herein, we examined the expression of AKT1, p‐AKT1, GSK3β and p‐GSK3β proteins in myocardial specimens via western blot (Figure 7A). Our data demonstrated that cardiac AKT1 (Figure 7B), p‐AKT1 (Figure 7C), GSK3β (Figure 7D) and p‐GSK3β (Figure 7E) proteins exhibited markedly higher expression in the DM +I/R group than the control +I/R group. Yet, their expression was lessened by AZA pretreatment in diabetic rats under I/R. Furthermore, we examined cardiac AKT1, p‐AKT1, GSK3β and p‐GSK3β proteins by immunohistochemistry (Figure 7F). Consistently, under I/R, higher AKT1 (Figure 7G), p‐AKT1 (Figure 7H), GSK3β (Figure 7I) and p‐GSK3β (Figure 7J) expression was detected in diabetic heart than controls. Their expression was markedly lessened by AZA pretreatment. Taken together, AZA pretreatment may decrease cardiac AKT1/GSK3β in diabetic rats under I/R.
FIGURE 7.

The effects of AZA pretreatment on cardiac AKT1/GSK3β in diabetic rats following I/R. (A) Representative images of western blot. Evaluation of (B) AKT1, (C) p‐AKT1, (D) GSK3β and (E) p‐GSK3β expression in heart tissues of the sham, control +I/R, DM +I/R and DM +I/R + AZA groups. (F) Immunohistochemistry results of heart sections. Bar = 20 μm. Determination of (G) AKT1, (H) p‐AKT1, (I) GSK3β and (J) p‐GSK3β expression in heart tissues of each group. **p < 0.01; ***p < 0.001 and ****p < 0.0001. AZA, azathioprine; DM, diabetes mellitus; I/R, ischaemia reperfusion
2.7. AZA pretreatment lessens cardiac Caspase8, GSK3β and p‐GSK3β expression in diabetic rats following I/R
Immunofluorescence also showed that Caspase8 (Figure 8A,B), GSK3β (Figure 8C) and p‐GSK3β (Figure 8D) had elevated expression in diabetic hearts than controls following I/R. But AZA pretreatment markedly lessened cardiac Caspase8, GSK3β and p‐GSK3β expression in diabetic rats following I/R.
FIGURE 8.

Immunofluorescence for the effects of AZA pretreatment on cardiac Caspase8, GSK3β and p‐GSK3β expression in diabetic rats following I/R. (A) Immunofluorescence of heart sections. Detection of cardiac (B) Caspase8, (C) GSK3β and (D) p‐GSK3β expression in the sham, control +I/R, DM +I/R and DM +I/R + AZA groups. **p < 0.01; ***p < 0.001 and ****p < 0.0001. AZA, azathioprine; DM, diabetes mellitus; I/R, ischaemia reperfusion
3. DISCUSSION
Cardiovascular complications are a common cause of death in diabetic patients. 16 Studies have found that continuous hyperglycaemia can not only induce cardiovascular ischaemic lesions and cause myocardial ischaemic damage, but also further aggravate ischaemic myocardial damage. 17 Therefore, looking for new interventions to reduce cardiac I/R damage in the diabetic state has important clinical and practical significance. In this study, we constructed a diabetic rat model with I/R operation. AZA pretreatment could ameliorate myocardial I/R injury in diabetic rats by reducing oxidative stress, apoptosis, and inflammation.
Our ECG results showed that AZA pretreatment improved ST segment downward shift and T wave inversion in diabetic rats following I/R, indicating that AZA pretreatment could ameliorate cardiac function under I/R. 18 After I/R, myocardial injury is one of the factors leading to heart dysfunction. 19 During myocardial I/R, myocardial cells secrete excessive collagen fibres, increase myocardial stiffness, slow down myocardial blood supply and further aggravate myocardial ischaemia. 20 Our data suggest that AZA pretreatment lessened myocardial infarction size and myocardial injury in diabetic rats with I/R.
Azathioprine pretreatment lowered the expression of myocardial injury markers (CK‐MB and MPO) and oxidative stress factors (MDA and SOD) in diabetic rats with I/R. In myocardial I/R injury, oxygen free radicals are the direct cause of I/R injury. The production of oxygen free radicals can damage the myocardial cell membrane, destroy the structure of myocardial cells and produce a large amount of lipid peroxides. 21 MDA is the product of lipid peroxidation of oxygen free radicals, and SOD is the main enzyme system for scavenging oxygen free radicals in the body. 22 Therefore, MDA can reflect the oxygen free radical content and lipid peroxidation index. SOD can be used to reflect the body's ability to resist lipid peroxidation. Our data showed that AZA pretreatment reduced serum MDA and SOD levels in diabetic rats with I/R, thereby ameliorating myocardial damage.
Previous studies have shown that in the early stage of myocardial blood supply interruption, cardiomyocyte apoptosis and inflammatory response begin to start. 23 Restoring myocardial blood perfusion can further aggravate cardiomyocyte apoptosis and activate the inflammatory cascade. 24 Inhibition of cardiomyocyte apoptosis and inflammation can continuously reduce myocardial damage induced by I/R, improve cardiac systolic and diastolic function, reduce myocardial infarction, and inhibit the occurrence and development of heart failure. 25 It is well acknowledged that apoptosis and inflammatory response usually occur in the diabetic heart with I/R injury. 26 Our findings demonstrate that AZA pretreatment decreased Caspase8, Caspase3, BAX, TLR4 and TNF‐α expression, and increased Bcl2 expression in myocardial tissues of diabetic rats following I/R, confirming the therapeutic effects of AZA pretreatment on diabetic cardiomyocyte apoptosis and inflammation under I/R. After I/R, the oxidative stress further activates the apoptotic and inflammatory factor TLR4, which affects the downstream Bcl‐2 and Bax expression, thereby enlarging the area of myocardial infarction. 27 Furthermore, pro‐inflammatory cytokine TNF‐α is an important cause of I/R, and its expression level can reflect the secondary inflammatory response after I/R. 28 The pro‐inflammatory cytokine TNF‐α released in the early stage of myocardial ischaemia can activate the inflammatory cascade in the inflammatory response stage, leading to the synthesis of inflammatory mediators and interleukins, and promoting the further release of macrophages, T cells, etc., thereby inducing heart damage. 29 Combining previous studies, AZA pretreatment could reduce cardiomyocyte apoptosis and inflammation in diabetic rats with I/R.
There is evidence that AKT1/GSK3β activation may facilitate cardiomyocyte apoptosis and inflammation following I/R. 15 , 30 , 31 Herein, diabetic rats were more vulnerable to AKT1/GSK3β activation after I/R operation. But AZA pretreatment lessened cardiac AKT1/GSK3β activation in diabetic rats with I/R. These findings indicated that AZA pretreatment improved myocardial I/R damage in diabetic rats by reducing oxidative stress, apoptosis, and inflammation, which could be related to AKT1/GSK3β inactivation.
Collectively, this study established an STZ‐induced diabetic rat model with I/R. AZA pretreatment improved cardiac function and ameliorated myocardial damage in diabetic rats through anti‐oxidative stress, anti‐apoptosis, and anti‐inflammatory response partly via AKT1/GSK3β pathway. However, more studies are required to confirm the therapeutic effects of AZA pretreatment on diabetic myocardial I/R damage.
4. METHODS
4.1. Animals and groups
Totally, 24 male Sprague‐Dawley rats weighing 200–220 g (Charles River Company; https://www.vitalriver.com/) were housed in an environment with a temperature of 22 ± 2°C, a relative humidity of 55 ± 5% and 12 h day/night cycle. All rats drank and ate freely. This study strictly followed the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. This animal experiment was approved by the Ethics Committee of Sichuan Vocational College of Health and Rehabilitation (2019026). The experiment was carried out after 1 week of adaptive feeding. All animals were divided into four groups (6 animals in each group): control + sham operation (sham); control +I/R; DM +I/R and DM +I/R + AZA.
4.2. Diabetic rat model
After fasting for 12 h, the rats were injected intraperitoneally with 60 mg/kg STZ. After 3 days, the fasting blood glucose of the rats was measured with a blood glucose meter. A fasting blood glucose concentration greater than 16.8 mmol/L indicated that the diabetic rat model had been successfully constructed.
4.3. Azathioprine pretreatment and myocardial I/R rat model
Diabetic rats were given 3 mg/kg AZA (HY‐B0256; MedChemExpress Company) daily by gavage for 5 days. 32 After that, the myocardial I/R rat model was established. SD rats were anaesthetised by intraperitoneal injection of 3% sodium pentobarbital at a dose of 50 mg/kg. After the limbs were fixed, an electrocardiogram (ECG) monitor was installed, followed by tracheal intubation and connection to an animal ventilator (tidal volume, 1.5 mL/100 g; frequency, 60 times/min; AE02‐ALC‐V8S; Shanghai Alcott Biotechnology Co., Ltd.). The ECG of the rats was recorded with needle electrodes. Afterwards, the rat skin was incised, starting from the flat line of the sternoclavicular joint and ending at the top of the xiphoid process based on the midline of the sternum. After bluntly separating muscles, the second to fourth ribs at the left edge of the sternum were cut off. By the chest opener, the pericardium was cut open to expose the heart. The left anterior descending coronary artery was separated, and 2/0‐T thread was used to ligate the left anterior descending coronary artery 1–2 mm. A concave latex tube was placed between the blood vessel and the ligature. The rats in the sham operation group remained unligated. Since myocardial ischaemia was displayed on the ECG, the ligature was cut off along the concave latex tube to restore myocardial blood flow after 30 min, and then kept for 120 min for reperfusion. During the ligation of the coronary artery, ECG changes were used to determine the success of the ischaemia–reperfusion model. The criteria were as follows: the left anterior descending coronary artery was ligated, and the ST segment was significantly elevated. During reperfusion, the ST segment rose and then fell, and the fall was not less than 50%.
4.4. Physiological index assessment
Before and after the establishment of the diabetic model, 5 days after administration of AZA and after the establishment of the I/R model, body weight and blood glucose of each rat were measured.
4.5. Neurological score
Neurological function of rats was assessed via the Berderson scoring method. 33 The rat's tail was suspended for 1 min, and the forelimb flexion was observed. The scoring criteria were as follows. 0 point, Normal rats stretched their upper limbs to the ground without symptoms of nerve injury. 1 point, The contralateral front paw cannot be fully extended in the tail suspension test. 2 points, The forelimb decreased ability to resist the contralateral thrust. 3 points, The forelimb made a circle to the opposite side.
4.6. Determination of myocardial infarction size
The 2,3,5‐Triphenyltetrazolium chloride (TTC; Solarbio) staining method was used to measure the myocardial infarction size in the isolated hearts of rats in each group. After the experiment, the heart was removed and placed in a refrigerator at −20°C for 5 min. Then, the whole heart was sliced with a thickness of 2–3 mm. The sections were placed in a 1% TTC solution dissolved in 0.1 mol/L PBS and kept in a water bath at 37°C for 15 min in the dark. Afterwards, the samples were fixed with 10% paraformaldehyde for 30 min before image acquisition.
4.7. H&E staining
The rat heart fixed in 4% paraformaldehyde was removed and dehydrated in different concentrations of alcohol. Then, the dehydrated sample was embedded in paraffin. Continuous dyeing was presented according to the instructions of H&E Staining Kit (Solarbio, #G1120). The stained myocardial sample was scanned with a digital slice scanner (Aperio VERSA; Leica).
4.8. Enzyme‐linked immunosorbent assay
Alanine aminotransferase (ALT; C009‐2‐1), aspartate aminotransferase (AST; C010‐2‐1), creatine kinase isoenzyme (CK‐MB; E006‐1‐1), malonaldehyde (MDA; A003‐1‐2), myeloperoxidase (MPO; A044‐1‐1) and superoxide dismutase (SOD; A001‐3‐2) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Serum ALT, AST, CK‐MB, MDA, MPO and SOD levels were then detected via enzyme‐linked immunosorbent assay (ELISA) methods.
4.9. Western blot
Myocardial tissues were added to RIPA lysate (Beyotime). After ultrasonic lysis, the supernatant was collected by centrifugation. The BCA method determined the protein concentration. The sample was separated by SDS‐PAGE electrophoresis and transferred to PVDF membrane (IPVH00010; Millipore). The membrane was blocked by 5% skimmed milk powder at room temperature, and incubated by the corresponding primary antibodies against Caspase8 (1:1000, 13423‐1‐AP; Proteintech), Caspase3 (1:500, 19677‐1‐AP; Proteintech), BAX (1:1000, 50599‐2‐Ig; Proteintech), Bcl2 (1:1000, 12789‐1‐AP; Proteintech), TLR4 (1:1000, 19811‐1‐AP; Proteintech), TNF‐α (1:1000, sc‐52746; Santa Cruz), AKT1 (1:1000, 10176‐2‐AP; Proteintech), Phospho‐AKT1 (Ser473) (1:500, 66444‐1‐Ig; Proteintech), GSK3β (1:1000, 22104‐1‐AP; Proteintech), Phospho‐GSK3β (Ser9) (1:1000, 67558‐1‐Ig; Proteintech) and β‐actin (1:1000, 20536‐1‐AP; Proteintech) at 4°C overnight. After washing the membrane, the secondary antibody (1:5000, #ZB‐2305, #ZB‐5301; ZSGB‐BIO) was used to incubate the membrane for 1 h on a shaker at room temperature. The sample was exposed after washing the film. Image J software was used for protein quantitative analysis.
4.10. Immunohistochemistry
Myocardial tissue was fixed with 4% paraformaldehyde. After dehydration with gradient ethanol, the sample was treated with xylene transparent and embedded in paraffin. Then, the sample was cut into 5 µm thickness. 0.1 mol/L sodium citrate buffer was used for high‐temperature antigen retrieval. After cooling to room temperature, the antigen was blocked with a 3% H2O2 methanol solution, and a hole was punched with triton X‐100. After rinsing with PBS, the section was incubated by primary antibodies against Caspase8 (1:100, 13423‐1‐AP; Proteintech), Caspase3 (1:80, 19677‐1‐AP; Proteintech), BAX (1:100, 50599‐2‐Ig; Proteintech), Bcl2 (1:100, 12789‐1‐AP; Proteintech), TLR4 (1:100, 19811‐1‐AP; Proteintech), TNF‐α (1:300, sc‐52746; Santa Cruz), AKT1 (1:100, 10176‐2‐AP; Proteintech), Phospho‐AKT1 (Ser473) (1:100, 66444‐1‐Ig; Proteintech), GSK3β (1:100, 22104‐1‐AP; Proteintech), Phospho‐GSK3β (Ser9) (1:100, 67558‐1‐Ig; Proteintech) overnight at 4°C, followed by incubation with HRP‐labelled secondary antibody (1:2000, ab205719; 1:3000, ab150077; Abcam) at 37 °C. Then, the nucleus was stained with hematoxylin. After dehydration, transparency and mounting, the images were observed under an optical microscope (Olympus).
4.11. Immunofluorescence
In brief, the section was incubated with primary antibodies against Caspase8 (1:100, 13423‐1‐AP; Proteintech) GSK3β (1:100, 22104‐1‐AP; Proteintech) and Phospho‐GSK3β (Ser9) (1:100, 67558‐1‐Ig; Proteintech) at 4°C overnight, followed by incubation with secondary antibodies including Alexa Fluor 488 Conjugate (1:100; #ZF‐0512; ZSGB‐BIO) and Alexa Fluor 594 Conjugate (1:100; #ZF‐0513) at room temperature for 2 h. The images were investigated under a fluorescence microscopy (Olympus). The expression of target proteins was quantified according to integrated optical density (IOD) value with ImageJ software.
4.12. Statistical analysis
Statistical analysis was presented via Graphpad Prism 8.0 (GraphPad Software). Each experiment was repeated ≥3. Data were expressed as mean ± standard deviation. Multiple comparisons were analyzed via one‐way analysis of variance followed by Tukey's test. p < 0.05 indicated statistical significance.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Cuijie Lu conceived and designed the study. Ling Liu, Shuai Chen and Junfei Niu conducted most of the experiments and data analysis, and wrote the manuscript. Sheng Li, Wenxian Xie and Xiang Cheng participated in collecting data and helped to draft the manuscript. All authors reviewed and approved the manuscript.
ACKNOWLEDGEMENTS
This work was funded by Key Science and Technology Plan Project of Zigong City (2019YLSF18).
Lu C, Liu L, Chen S, et al. Azathioprine pretreatment ameliorates myocardial ischaemia reperfusion injury in diabetic rats by reducing oxidative stress, apoptosis, and inflammation. Clin Exp Pharmacol Physiol. 2021;48:1621–1632. 10.1111/1440-1681.13569
Cuijie Lu, Ling Liu and Shuai Chen: Equal contributors
DATA AVAILABILITY STATEMENT
The raw data analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Xin C, Zhang Z, Gao G, et al. Irisin attenuates myocardial ischemia/reperfusion injury and improves mitochondrial function through AMPK pathway in diabetic mice. Front Pharmacol. 2020;11:565160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39(2):210‐225. [DOI] [PubMed] [Google Scholar]
- 3. Chien CY, Wen TJ, Cheng YH, Tsai YT, Chiang CY, Chien CT. Diabetes upregulates oxidative stress and downregulates cardiac protection to exacerbate myocardial ischemia/reperfusion injury in rats. Antioxidants. 2020;9(8):679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Qiu Z, Ming H, Lei S, et al. Roles of HDAC3‐orchestrated circadian clock gene oscillations in diabetic rats following myocardial ischaemia/reperfusion injury. Cell Death Dis. 2021;12(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma W, Guo W, Shang F, et al. Bakuchiol alleviates hyperglycemia‐induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/Nrf2 signaling pathway. Oxid Med Cell Longev. 2020;2020:3732718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiao C, Xia ML, Wang J, et al. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid Med Cell Longev. 2019;2019:2719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu LM, Dong X, Xue XD, et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia‐reperfusion injury by improving mitochondrial quality control: role of SIRT6. J Pineal Res. 2021;70(1):e12698. [DOI] [PubMed] [Google Scholar]
- 8. Zheng D, Cao T, Zhang LL, Fan GC, Qiu J, Peng TQ. Targeted inhibition of calpain in mitochondria alleviates oxidative stress‐induced myocardial injury. Acta Pharmacol Sin. 2020;42(6):909‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Penna C, Andreadou I, Aragno M, et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia‐reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br J Pharmacol. 2020;177(23):5312‐5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao D, Yang J, Yang L. Insights for oxidative stress and mTOR signaling in myocardial ischemia/reperfusion injury under diabetes. Oxid Med Cell Longev. 2017;2017:6437467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol. 2020;16(3):167‐178. [DOI] [PubMed] [Google Scholar]
- 12. Hernández‐Breijo B, Gomez A, Soukka S, Johansson P, Parodis I. Antimalarial agents diminish while methotrexate, azathioprine and mycophenolic acid increase BAFF levels in systemic lupus erythematosus. Autoimmun Rev. 2019;18(10):102372. [DOI] [PubMed] [Google Scholar]
- 13. Lucafò M, Stocco G, Martelossi S, et al. Azathioprine biotransformation in young patients with inflammatory bowel disease: contribution of glutathione‐S transferase M1 and A1 variants. Genes. 2019;10(4):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rebić N, Sayre EC, Zusman EZ, Amiri N, Baldwin C, De Vera MA. Perinatal use and discontinuation of disease‐modifying anti‐rheumatic drugs and biologics in women with rheumatoid arthritis: a cohort study. Rheumatology. 2020;59(7):1514‐1521. [DOI] [PubMed] [Google Scholar]
- 15. Ma L, Kerr BA, Naga Prasad SV, Byzova TV, Somanath PR. Differential effects of Akt1 signaling on short‐ versus long‐term consequences of myocardial infarction and reperfusion injury. Lab Invest. 2014;94(10):1083‐1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avogaro A, Fadini GP. Microvascular complications in diabetes: a growing concern for cardiologists. Int J Cardiol. 2019;291:29‐35. [DOI] [PubMed] [Google Scholar]
- 17. Wu L, Gunton JE. The changing landscape of pharmacotherapy for diabetes mellitus: a review of cardiovascular outcomes. Int J Mol Sci. 2019;20(23):5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang SC, Sohn EH, Lee SR. Hydrogen sulfide as a potential alternative for the treatment of myocardial fibrosis. Oxid Med Cell Longev. 2020;2020:4105382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao Y, Yang ZG, Ren Y, et al. Evaluation of myocardial fibrosis in diabetes with cardiac magnetic resonance T1‐mapping: correlation with the high‐level hemoglobin A1c. Diabetes Res Clin Pract. 2019;150:72‐80. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Wang T, Zhang M, Chen P, Yu Y. Down‐regulation of myocardial infarction associated transcript 1 improves myocardial ischemia‐reperfusion injury in aged diabetic rats by inhibition of activation of NF‐κB signaling pathway. Chem Biol Interact. 2019;300:111‐122. [DOI] [PubMed] [Google Scholar]
- 21. Kang PF, Wu WJ, Tang Y, et al. Activation of ALDH2 with low concentration of ethanol attenuates myocardial ischemia/reperfusion injury in diabetes rat model. Oxid Med Cell Longev. 2016;2016:6190504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu S, Chang G, Gao L, et al. Trimetazidine protects against myocardial ischemia/reperfusion injury by inhibiting excessive autophagy. J Mol Med. 2018;96(8):791‐806. [DOI] [PubMed] [Google Scholar]
- 23. Yao L, Chen H, Wu Q, Xie K. Hydrogen‐rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK‐mediated autophagy. Int J Mol Med. 2019;44(3):1048‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou X, Fu M, Cheng B, Kang Y, Xie D. Galanthamine improves myocardial ischemia‐reperfusion‐induced cardiac dysfunction, endoplasmic reticulum stress‐related apoptosis, and myocardial fibrosis by suppressing AMPK/Nrf2 pathway in rats. Ann Transl Med. 2019;7(22):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pei YH, Chen J, Wu X, et al. LncRNA PEAMIR inhibits apoptosis and inflammatory response in PM2.5 exposure aggravated myocardial ischemia/reperfusion injury as a competing endogenous RNA of miR‐29b‐3p. Nanotoxicology. 2020;14(5):638‐653. [DOI] [PubMed] [Google Scholar]
- 26. Lejay A, Fang F, John R, et al. Ischemia reperfusion injury, ischemic conditioning and diabetes mellitus. J Mol Cell Cardiol. 2016;91:11‐22. [DOI] [PubMed] [Google Scholar]
- 27. Zhang X, Du Q, Yang Y, et al. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF‐κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042‐1052. [DOI] [PubMed] [Google Scholar]
- 28. Dong Y, Chen H, Gao J, Liu Y, Li J, Wang J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27‐41. [DOI] [PubMed] [Google Scholar]
- 29. Sun X, Wei Z, Li Y, et al. Renal denervation restrains the inflammatory response in myocardial ischemia‐reperfusion injury. Basic Res Cardiol. 2020;115(2):15. [DOI] [PubMed] [Google Scholar]
- 30. Mersmann J, Tran N, Latsch K, et al. Akt or phosphoinositide‐3‐kinase inhibition reverses cardio‐protection in Toll‐like receptor 2 deficient mice. Resuscitation. 2012;83(11):1404‐1410. [DOI] [PubMed] [Google Scholar]
- 31. Gao HK, Yin Z, Zhou N, Feng XY, Gao F, Wang HC. Glycogen synthase kinase 3 inhibition protects the heart from acute ischemia‐reperfusion injury via inhibition of inflammation and apoptosis. J Cardiovasc Pharmacol. 2008;52(3):286‐292. [DOI] [PubMed] [Google Scholar]
- 32. Zhang C, Zhang M, Qiu W, et al. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open‐label, multicentre, randomised, phase 2 trial. Lancet Neurol. 2020;19(5):391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo M, Tang X, Zhu J, Qiu Z, Jiang Y. Establishment of acute pontine infarction in rats by electrical stimulation. J Vis Exp. 2020;162:60783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data analyzed during the current study are available from the corresponding author on reasonable request.


