Figure 1.
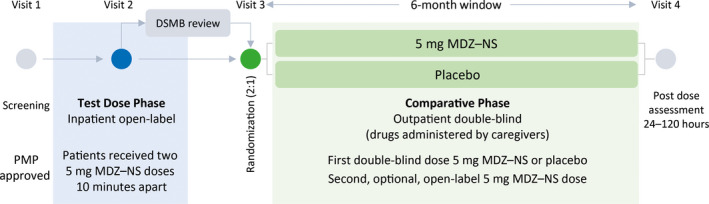
Trial design. At screening, each patient's individualized patient management plan (PMP) was reviewed by a member of the Epilepsy Study Consortium who determined whether the cluster description met the protocol definition and provided final approval for trial participation. A review of safety data from at least the first 25 patients who completed the TDP was required before patients could progress to the CP. The review was conducted by an independent Data and Safety Monitoring Board (DSMB), as were further reviews at periodic intervals during the trial. MDZ–NS, midazolam nasal spray
