Figure 2.
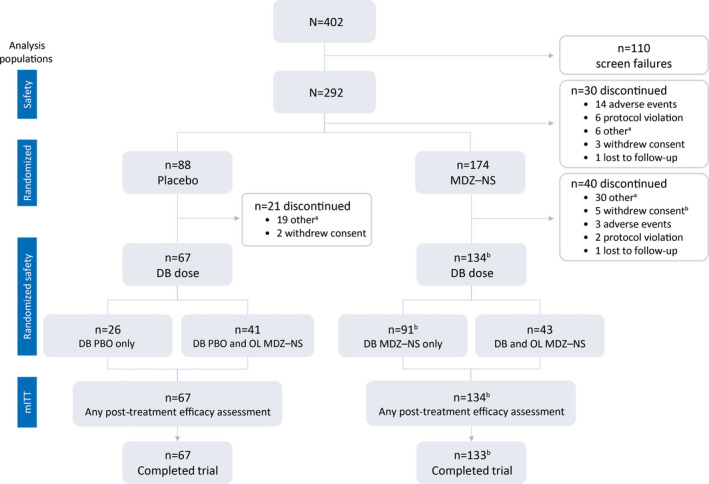
Patient disposition over the course of the trial and analysis populations. DB, double‐blind; MDZ–NS, midazolam nasal spray; mITT, modified intent‐to‐treat; OL, open‐label aOther reasons for discontinuation included: patient did not experience/treat seizure cluster(s) according to trial criteria within protocol‐specified time period; caregiver no longer available; trial drug unavailable at site; patient/caregiver unable to comply with trial procedures/visits; trial terminated; site closure bOne patient received treatment in the CP and had postdose efficacy assessments, but subsequently withdrew consent before completing procedures at visit 4
