Abstract
Objective
A 730 nm picosecond‐domain laser was developed to improve the clearance of pigmented lesion and reduce adverse events. This study assessed the safety and efficacy of this system for the clearance of lentigines and explores how the short picosecond pulses interact with tissue via histology.
Study Design and Methods
Twenty subjects with Fitzpatrick skin types II–IV were enrolled in this prospective, IRB‐approved study. Four treatments were administered using a 730 nm picosecond‐domain laser. Pre‐ and posttreatment photos were assessed by blinded reviewers at 4‐ and 12‐week follow‐up visits, using a 5‐point clearance scale. Subject satisfaction was measured using a 5‐point scale. Investigator Global Improvement Score (IGIS) was performed at the 4‐ and 12‐week follow‐up visits, using an 11‐point clearance scale. Subject pain level was measured using an 11‐point scale (no pain [0], extreme pain [10]). Histology of 730 and 532 nm picosecond pulses was compared with 755 and 532 nm nanosecond pulses.
Results
Sixteen subjects with a total of 118 discontinuous treatment areas, each comprised of 1–20 lesions, completed all study visits. Thirty body regions were studied, including arms (6), hands (16), scalp (1), forehead (2), face (3), and back (2). Spot sizes ranging from 2 to 5 mm diameters were used with fluences ranging from 0.8 to 4.0 J/cm2. Mean pain score was 3.6 of 10 for all four treatments. Ninety‐nine percent of randomly paired 4‐week posttreatment images and 100% of 12‐week posttreatment images were correctly identified from their respective baseline images by three blinded reviewers. Mean IGIS demonstrated scores of 6.7 and 7.0 at 4‐ and 12‐week follow‐up visits, respectively. At the 4‐ and 12‐week follow‐up visits, 76% and 73% of subjects, respectively, were satisfied to highly satisfied. The mean clearance score for all 118 treatment areas was 3 of 4 in follow‐up visits. At 12‐week follow‐up, 36% of 118 treatment areas had a clearance score of 4, and 38% had a clearance score of 3. Post treatment, there was typical erythema, edema, dryness, crusting, and itching but negligible purpura, no pinpoint bleeding, blistering or scarring, and no significant hyperpigmentation or hypopigmentation. Histology showed diffuse, focal epidermal vacuolization ~5–10 µm in diameter and mild extravasation of erythrocytes with 730 nm picosecond pulses, while diffuse epidermal vacuolization was observed with coalescence of vacuoles (~20–100 µm), junctional clefting and mild extravasation of erythrocytes with 755 nm nanosecond pulses. Picosecond pulses of the wavelength of 532 nm produced diffuse, focal epidermal vacuolization and larger dermal vacuoles to depths of 500 µm, while 532 nm nanosecond pulses produced diffuse epidermal vacuolization with coalescence of vacuoles and marked dermal hemorrhage.
Conclusion
This study demonstrated the potential of a new 730 nm picosecond‐domain laser for the clearance of lentigines. The results showed good clearance with no adverse events and good subject satisfaction in patients with skin type II–III. Additional studies need to be conducted on darker skin types. The histopathologic findings demonstrate that the picosecond 730 nm laser produces excellent selectivity for pigment with minimal disruption of the dermal–epidermal junction and may therefore reduce healing times and the risk of adverse events.
Keywords: histology, laser, lentigines, nanosecond, picosecond, pigment, Q‐switched, solar lentigo
INTRODUCTION
Solar lentigines are a common sign of photoaging and patients often seek their removal for aesthetic improvement. Solar lentigines result from long‐term, intermittent sun exposure and appear in increasing number with advancing age. They are well demarcated, light to dark brown macules, ranging in size from 2 mm to over 1 cm and appear on sun‐exposed skin. 1 Histologically, they are characterized by increased melanin in the basal layer, most with a mildly increased number of melanocytes in the basal layer of the epidermis and variable elongation of the rete ridges. Quality switched lasers with nanosecond domain pulses and millisecond domain intense pulse light sources have been commonly used for the treatment of lentigines for almost three decades. By choosing wavelengths that are preferentially absorbed by melanin, and pulse durations that confine heating to lesional skin, lasers and light sources selectively destroy these lesions without harming the surrounding skin. 2 , 3 Post‐inflammatory hyperpigmentation (PIH) may occur following treatment, particularly in darker phototype skin, at a reported rate of 10%–47%. 4 , 5 , 6 , 7 , 8 , 9
In recent years, picosecond lasers were developed with the goal of optimizing tattoo treatments because of their ability to better target smaller particles the size of tattoo granules. 10 , 11 Treatment with picosecond pulses produces faster clearance of tattoo pigment, and results in shorter treatment recovery times and fewer overall adverse side effects. 12 , 13 , 14 These lasers have also proven useful for the treatment of benign pigmented lesions, and, when delivered in a fractionated mode, for the treatment of photoaging and acne scars. 9 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Compared with nanosecond pulses, picosecond pulses produce relatively more photomechanical effects over photothermal effects and achieve better heat confinement, and therefore may reduce the risk of PIH when used for treating benign pigmented lesions. A novel laser pumped 730 nm titanium–sapphire (Ti:sapphire) laser has been developed that effectively removes multicolored tattoos comprising black, blue, green, and purple inks. 22 Herein, we report the results of the first prospective trial examining the use of the 730 nm picosecond laser for the treatment of lentigines located on the face, trunk, and extremities.
MATERIALS AND METHODS
This study is a prospective, open label, evaluator‐blinded clinical trial to assess the safety and efficacy of the 730 nm Ti:sapphire laser‐pumped picosecond laser (PicoWay®; Candela Medical) for the treatment of benign pigmented lesions. The protocol was reviewed and approved by an independent review board (Chesapeake IRB) for the treatment of human subjects, and written informed consent was obtained from all the subjects before treatment.
Subjects
Male and female subjects with skin phototypes I–IV and lentigines present on the face, trunk or extremities, ages 18–70 years, were recruited for this study. Subjects with the following conditions were excluded from the study: pregnancy or lactation; allergy to topical or injectable anesthetics; allergy to topical corticosteroids; active sun tan; history of melanoma; history of hyperpigmentation; history of vitiligo; history of keloid or hypertrophic scar formation; use of systemic retinoid therapy during the past 12 months; immunosuppression; open wound or infection in the intended treatment area; history of light‐induced seizure disorders; and surgical, light, laser, or radiofrequency procedures in the intended treatment area during the past 3 months. The study allowed for optional biopsies to assess the immediate histological effects of 730 nm picosecond laser treatment of lentigines while comparing to treatment effects of 532 nm picosecond pulses and 532 and 755 nm nanosecond pulses.
Study device
The device used in this study was a laser‐pumped 730 nm picosecond laser. It consisted of a Ti:sapphire crystal placed in an accessory handpiece for use with a commercial picosecond domain system (PicoWay) that is equipped with four wavelengths: 1064, 785, 730, and 532 nm. In this laser system, the frequency‐doubled 532 nm Nd:YAG (neodymium‐doped yttrium aluminum garnet) laser pumps the Ti:sapphire crystal to deliver 730 nm pulses with a maximum energy of 100 mJ and a pulse duration of 246 ps. Laser beam diameters range from 2 to 6 mm, in 1 mm increments, with fluences of 0.6–4.0 J/cm2 at 1–10 Hz.
Laser treatment
The skin was cleansed with 70% isopropyl alcohol. Subjects were given the option of no anesthesia or topical anesthesia. A maximum of four treatments was permitted with treatments spaced 4 weeks apart. The treatment parameters were chosen by the investigator based on subject's skin type, the clinical endpoint, and the investigator's experience. The desired endpoint was slight tissue whitening or erythema immediately after laser application. Ice packs and triamcinolone cream 0.1% were applied immediately following treatment. Patients were advised to use a bland moisturizer until the treated areas healed and a broad‐spectrum sunscreen, with an SPF 40 or higher for the duration of the study.
Blinded evaluation of digital images
Before each treatment session and at every follow‐up visit, the treatment areas were photographed using standardized positioning and lighting conditions, with a high‐resolution digital camera imaging system (Canfield IntelliStudio; Canfield Scientific).
At the completion of the study, two types of blinded photographic evaluation were performed by three dermatologists who were not involved in the laser treatments. In the first evaluation, the pre‐ and posttreatment photograph sets were randomized to the right and left panel in a PowerPoint presentation (Microsoft Corporation) for blinded scoring by the evaluators. As the entire region was not treated, the treated areas were mapped and circled in each photo to aid in the identification of the treated lesions, to avoid the influence of nearby lesions that were not treated. All photographic images were globally graded using a 5‐point scale (0–4): A score of 0 indicating 0% clearance, 1 indicating 1%–24% clearance (poor response), 2 indicating 25%–49% improvement (moderate response), 3 indicating 50%–74% clearance (good response), and a score of 4 indicating 75%–100% clearance (excellent response). If a reviewer were to incorrectly identify a baseline photo, the score would be recorded as the negative of the given score.
The second analysis consisted of the designated pre‐ and posttreatment photographic images placed side‐by‐side, with the baseline image on the left and follow‐up on the right in a PowerPoint file. For this assessment, each treatment area was individually assessed resulting in 113 (blinded to treatment parameters) scores at 4‐week follow‐up, and 118 (blinded to treatment parameters) scores at 12‐week follow‐up. The same 5‐point scale as above was used.
Investigator Global Improvement Score (IGIS)
The IGIS was performed at 4 weeks and 12 weeks, using an 11‐point clearance scale (0–10). A score of 0 indicates no improvement, score of 1 indicates 10% improvement, up to a score of 10 for 100% improvement (complete clearance).
Subject Satisfaction Score (SSS)
Subject satisfaction was assessed after each study visit following the initial treatment. The SSS was measured using a 5‐point scale with a score of −2 indicating highly dissatisfied, −1 indicating dissatisfied, 0 indicating neither satisfied nor dissatisfied, 1 indicating satisfied, and 2 indicates highly satisfied.
Histological analysis
Optional biopsies were taken for histological confirmation studies in a subset of the study population. Biopsies were performed immediately after laser treatment of lentigines in an area distinct from those treated for the clinical portion of the study. Lentigines were treated with a nanosecond 532 nm Nd:YAG laser with a 3 mm spot size and fluence of 1.8 J/cm2 (Alex Trivantage; Candela Medical), a 532 nm picosecond laser with a 3–4 mm spot size and fluence of 1–1.2 J/cm2 (PicoWay), a nanosecond 755 nm laser with a 3 mm spot and fluence of 7–7.5 J/cm2 (Alex Trivantage) and the picosecond Ti:sapphire 730 nm laser with a 2–3 mm spot size and fluence of 1.8–4 J/cm2. Single pulses were applied with a clinical endpoint of skin whitening. Immediately after treatment, the lesions were anesthetized with 1% lidocaine with 1:100,000 epinephrine and removed with 3 mm punch biopsies. The biopsies were step‐sectioned and stained with hematoxylin and eosin for evaluation with light microscopy.
Side effects
The clinical safety endpoint was characterized by the observation of immediate whitening or erythema of the treated lesions. Local skin responses following treatment were assessed and recorded by the investigator 10–15 min after the treatment. The anticipated side effects were as follows: erythema, edema, petechiae, pinpoint bleeding crusting, or scabbing. The occurrence of adverse events including PIH, hypopigmentation, vesiculation, burns, and scars was recorded during the follow‐up visits. Treatment safety was assessed by evaluating these findings at each study visit following the initial treatment, using a 4‐point scale where 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. The subject's pain level was evaluated using an 11‐point analog scale, where 0 indicates no pain and 10 indicates extreme pain.
Statistical analysis
Analyses were performed using commercial software (Excel; Microsoft). Results from blinded assessment, subject pain score, IGIS, SSS were reported as means with standard errors (SEM).
RESULTS
Twenty subjects with 37 treated body regions were enrolled in this study and 16 subjects with 30 treated body regions completed the study protocol. Four subjects discontinued treatment due to reasons unrelated to the study. Among the remaining 16 subjects, the mean age was 63, with a range of 53–70 years of age. Seventy‐five percent (n = 12) of completed subjects were female and 25% were male (n = 4). Eighty‐eight percent of subjects had Fitzpatrick skin type II, and 12% had Fitzpatrick skin type III (Table 1).
Table 1.
Subjects characteristics
| 20 subjects enrolled in the study (37 body sites) | |
| 16 subjects completed the study (30 body sites and 118 treatment areas) | |
| Mean age (range) | 63 (53–70) |
| Gender | |
| Male | 4 (25%) |
| Female | 12 (75%) |
| Skin type | |
| II | 14 (87.5%) |
| III | 2 (12.5%) |
| Race/ethnicity | |
| Hispanic or Latino | 1 (6.25%) |
| Caucasian | 15 (93.75) |
| Body areas | |
| Arm | 6 (20%) |
| Hand | 16 (53.3%) |
| Scalp | 1 (3.3%) |
| Forehead | 2 (6.6%) |
| Face | 3 (9.9%) |
| Back | 2 (6.6%) |
The first laser treatment session was performed with an average beam diameter of 2.9 mm and fluence of 2.2 J/cm2. During the subsequent treatment sessions, laser fluences were successively increased (mean fluence of 3.1, 3.4, and 3.9 J/cm2 for the second, third, and fourth treatment sessions, respectively), to achieve a clinical endpoint of tissue whitening with minimal pulse overlap. This necessitated a reduction in the spot size, with mean spot sizes decreasing from 2.9 mm for the first treatment session to 2.0 mm for the fourth treatment session (Figure 1A,B and Table 2). Fifteen of sixteen subjects received four treatments at 4‐week intervals and returned for 4‐ and 12‐week follow‐up visits. One subject received three treatments and returned only for the 12‐week follow‐up visit due to personal scheduling conflicts unrelated to the study. The treatments were tolerated well by the subjects, with only 1 of 16 subjects receiving topical anesthesia and another 2 of 16 subjects using a cold roller immediately after treatment (Table 2). The average pain score for all sessions was 3.6 (±0.2) of a maximum pain sore of 10 (Figure 2).
Figure 1.
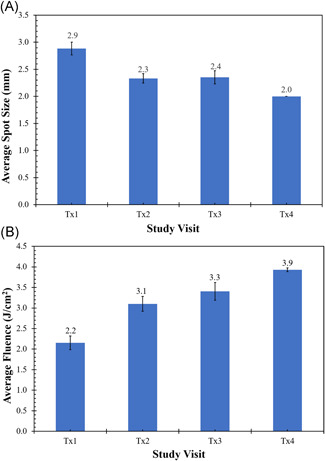
Treatment parameters versus treatment session. (A) Spot size versus treatment session. (B) fluence versus treatment session
Table 2.
Treatment parameters for each laser treatment
| Parameter | Tx1 | Tx2 | Tx3 | Tx4 | |
|---|---|---|---|---|---|
| Fluence (J/cm2) | |||||
| Mean | 2.2 | 3.1 | 3.4 | 3.9 | |
| SD | 1.0 | 1.0 | 1.2 | 0.3 | |
| Min | 1.0 | 1.8 | 0.8 | 3.0 | |
| Max | 4.0 | 4.0 | 7.5 | 4.0 | |
| Spot size range (mm) | 2 to 4 | 2 to 3 | 2 to 5 | 2 to 2 | |
| Reptition rate range (Hz) | 2 to 5 | 3 to 5 | 4 to 8 | 3 to 8 | |
| No. of Tx | 34 | 30 | 34 | 29 | |
| Anesthesia | |||||
| Topical | 2 | 2 | 0 | 2 | |
| Ice roller | 1 | 0 | 2 | 2 | |
| None | 31 | 28 | 32 | 25 | |
| Pain score | |||||
| Mean | 2.9 | 3.8 | 3.5 | 4.5 | |
| SD | 1.9 | 2.3 | 2.2 | 2.6 | |
| Min | 0.0 | 0.0 | 1.0 | 1.0 | |
| Max | 7.0 | 8.0 | 8.0 | 8.0 | |
Figure 2.
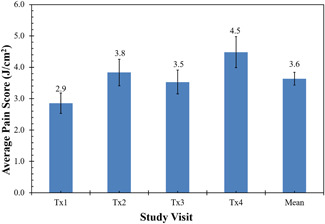
Mean pain scores for each treatment session and for all treatment sessions combined
Blinded evaluation of digital images
Among the three blinded evaluators, there was only one image at the 4‐week follow‐up that was incorrectly identified by one evaluator as a posttreatment photograph. Therefore, 99% of 4‐week posttreatment images, 100% of 12‐week posttreatment images, were correctly identified.
The average global clearance score for all follow up visits on a 5‐point scale from 0 (no improvement) to 4 (complete clearance) was 3.0 (±0.1), corresponding to 51%–75% improvement at all follow‐up visits. Figures 3 and 4 demonstrate good correlation of global clearance scores among the three blinded dermatologist evaluators. Among all 113 treatment areas at the 4‐week follow‐up, 33% achieved a clearance score of 4 (75%–100% clearance), 41% had a score of 3 (50%–74% clearance), 16% had a score of 2 (25%–49% clearance), 9% had a score of 1 (1%–24% clearance), and 1% had a score of 0. No cases were recorded as a negative score in the assessment of 113 treatment areas. Among all 118 areas at 12‐week follow‐up, 36% had a score of 4 (75%–100% clearance), 38% had a score of 3 (50%–74% clearance), 20% had a score of 2 (25%–49% clearance), and 6% had a score of 1 (1%–24% clearance). A similar level of clearance was observed in all treatment areas at the 12‐week follow‐up visit. On average, 74% of treatment areas achieved ≥50% clearance at 4‐week post treatment and 73% treatment areas achieved ≥50% clearance at 12‐week post treatment. Figure 5 shows typical treatment results on the back, face and hand.
Figure 3.
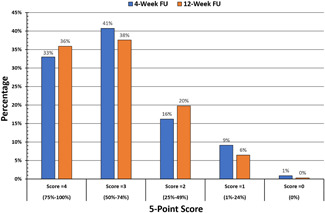
Distribution of clearance scores at the 4‐ and 12‐week follow‐up visits
Figure 4.
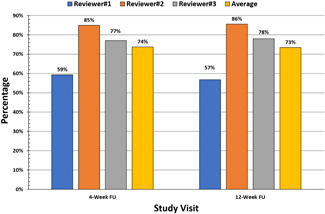
Percentage of treatment areas achieving >50% clearance by blinded assessment
Figure 5.
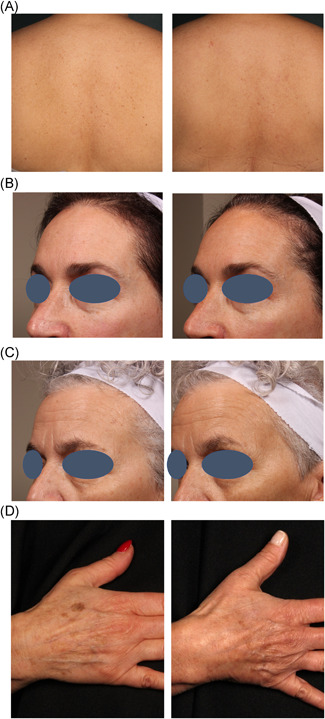
Baseline and 12‐week follow‐up with a 730 nm picosecond laser on the (A) back. (B,C) face. (D) hand
SSS and IGIS
The SSS was recorded at treatment visits 2, 3, and 4, 4‐week and 12‐week follow‐up visits using the 5‐point scale (−2 to 2). Before the second treatment, 67% of subjects felt satisfied or highly satisfied. This number increased to 83% at treatment 3 and 90% at treatment 4. The SSS was 76% at 4‐week follow‐up and 73% at 12‐week follow‐up. The investigator evaluated the 4‐ and 12‐week follow‐up photos using an 11‐points IGIS. The 4‐week IGIS was 6.7 of 10, and the 12‐week IGIS was 7.0 of 10.
Side effects
As anticipated, erythema, edema, and crusting developed after treatment. The side effect profile following treatments was as follows: mild to moderate erythema (93%); mild to moderate edema (79%); mild to moderate crusting (33%); mild dryness (28%); mild pruritus (6%) and mild purpura (2%).
Mild PIH developed in 4 of the 20 enrolled subjects after the first or second treatment session and resolved within 1 month. The PIH occurred in just one or two lesions in each subject of a total of over 1000 lesions treated in the study, and there was no occurrence of PIH after the third or fourth treatment session and no occurrence of hypopigmentation or scarring. The PIH resolved within 3–4 weeks of using fluocinolone acetonide 0.1% and 4% hydroquinone cream.
Histological analysis
Nine biopsies were taken from three subjects. Three biopsies were taken immediately following treatment with 730 nm picosecond pulses (#105 right upper back, #112 left upper arm, and #114 left abdomen) and 755 nm nanosecond pulses (#105 left upper back, #122 right upper arm, and #114 right abdomen); two biopsies were taken following treatments with 532 nm picosecond pulses (#112 left upper arm and #114 left upper back), and a single biopsy following treatments with 532 nm nanosecond pulses (#114 right upper back). Figure 6 shows the histological findings of lentigines following application of 730 and 532 nm picosecond pulses, and 755 and 532 nm nanosecond pulses.
Figure 6.
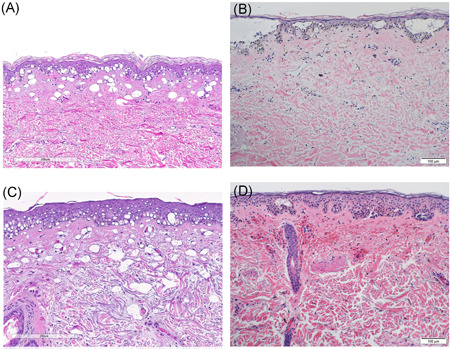
(A) Histologic findings after treatment of a solar lentigo with the 730 nm picosecond laser (2 mm spot, 4 J/cm2). (B) Histologic findings after treatment of a solar lentigo with the nanosecond 755 nm laser (3 mm spot, 7 J/cm2). (C) Histologic findings after treatment of a solar lentigo with the 532 nm picosecond laser (4 mm spot, 1.2 J/cm2). (D) Histologic findings after treatment of a solar lentigo with the nanosecond 532 nm laser (3 mm spot, 1.8 J/cm2)
Treatment with the 730 nm picosecond laser produced vacuolar degeneration of the lower layer of epidermal keratinocytes as well as papillary dermal vacuoles (Figure 6A), congested vessels, mild red blood cell extravasation in the upper dermis, and a moderate superficial perivascular lymphocytic infiltrate. Treatment with the nanosecond 755 nm laser produced extensive vacuolar degeneration of the lower layer of epidermal keratinocytes coalescing to form intraepidermal vesicles, dermal epidermal clefting, a sparse superficial perivascular lymphocytic infiltrate, and mild extravasation of erythrocytes in the upper dermis (Figure 6B).
Treatment with the 532 nm picosecond laser produced extensive vacuolar degeneration of lower layer of epidermal keratinocytes. Vacuoles were also present in the reticular dermis (Figure 6C). The nanosecond 532 nm laser produced focal vacuolar degeneration of lower layer of epidermal keratinocytes with intra‐epidermal vesiculation and marked upper dermal hemorrhage (Figure 6D).
DISCUSSION AND CONCLUSION
Picosecond laser technology was initially developed for commercial use to improve tattoo clearance by means of fracturing nanometer‐sized tattoo ink particles aggregated within macrophages and fibroblasts more efficiently than nanosecond domain lasers. 10 , 11 Multiple clinical studies have substantiated these claims with multicolored tattoos clearing in fewer treatments than with nanosecond‐domain lasers. 12 , 13 , 14 Recovery time is shorter and there are fewer adverse events because the laser–tissue interaction is more photomechanical than photothermal, and there is better confinement of thermal damage with picosecond domain pulses. In recent years, picosecond lasers, including the 755, 532, and 1064 nm wavelengths, were shown to be effective for the removal of benign epidermal and dermal pigmented lesions, including solar lentigines, freckles, nevus of Ota, and melasma with a relatively low risk of adverse events. 9 , 17 , 23 However, PIH remains a concern when treating epidermal pigmentation with any short‐pulsed laser, particularly in darker phototype skin, with a reported risk of 10%–47%. 4 , 5 , 6 , 7 , 8 , 9 We theorized that the 730 nm Ti:sapphire picosecond laser may provide a higher safety profile than the 532 nm wavelength because of its lower absorption coefficient for melanin, which should diminish absorption by non‐lesional epidermal melanin; and its lower absorption by hemoglobin which should reduce the risk of pinpoint bleeding and purpura. Additionally, the primarily photomechanical effects observed with picosecond lasers in tissue should confer additional safety compared with nanosecond domain lasers by reducing thermal effects in tissue. The Ti:sapphire 730 nm wavelength is the fourth wavelength that was added to a picosecond domain laser system that includes 1064, 785, and 532 nm wavelengths. The crystal is housed in a handpiece cartridge that is attached to the laser's articulated arm and is pumped by the frequency‐doubled 532 nm picosecond laser. The picosecond Ti:sapphire 730 nm laser was recently shown to be effective for treating blue, purple, green, and black tattoo inks in Fitzpatrick skin types I–III. 22
The present study demonstrates the safety and efficacy of the Ti:sapphire 730 nm picosecond laser for the treatment of facial and non‐facial lentigines. Three blinded investigators correctly chose randomized before and after images of the treated lentigines with 99% accuracy at 4‐week follow‐up, and 100% accuracy at 12‐week follow up. The mean improvement for all treatment areas was a 3.0 (±0.1), representing 51%–75% clearance. Results were durable with mean clearances of 3.0 (±0.9) at 4‐week versus 3.0 (±0.9) at 12‐week follow‐up. The treatment was well tolerated with only one subject requesting pretreatment topical anesthesia and the mean treatment pain score for all subjects was 3.6 out of 10. As anticipated, erythema, edema, crusting, and a low incidence of vesiculation occurred following treatment, and the side effects resolved within 3–5 days.
Our results compare favorably with previous reports of picosecond lasers used for treating solar lentigines. In a retrospective analysis of a 750 ps, 755 nm laser used to treat flat and raised pigmented lesions in phototype I–III skin, 23 there was a mean clearance of 61% in flat lesions. In a prospective trial of a picosecond versus a nanosecond 532 nm laser for paired upper extremity lesions in phototype III and IV Asian skin, 68% of subjects had >75% clearance of the lesions treated with the picosecond laser and 71% with the nanosecond laser at the 12‐week follow‐up. 24 PIH occurred in 7.1% of subjects and hypopigmentation in 3.6% of subjects. Chan et al. 25 performed a prospective study of 20 subjects with 89 solar lentigines with a 750 ps, 532 nm laser and found 40% of subjects achieved moderate improvement and 50% of subjects achieved significant improvement, using an investigator global assessment score; PIH developed in five subjects and hypopigmentation in one subject, with all resolved by the 12‐week follow‐up visit. Negishi et al. 26 studied 43 lesions of facial solar lentigines in 20 subjects with phototype III and IV Asian skin using a 750 ps, 532 nm laser. At the 12‐week follow‐up visit, 77% of lesions had 76%–100% clearance and 14% of lesions had 51%–75% clearance by investigator assessment; PIH occurred in 5% of subjects and prolonged erythema in 5% of subjects, but no occurrence of hypopigmentation.
Lee et al. 27 via case reports on two subjects were the first to report successful treatment of pigmentary lesions following a single treatment with the 730 nm Ti:sapphire picosecond laser. In the one subject treated for solar lentigines, 43% of the lesions achieved an excellent response and 29% of the lesions achieved a good response. No PIH was observed.
Lipp et al. performed a retrospective review of their experience with the Ti:sapphire 730 ps laser for pigmented lesions in phototype II–VI skin. The mean beam diameter used was 2.2 mm and the mean fluence was 3.4 J/cm2. Of the 22 patients treated, 15 had solar lentigines. Thirty‐one percent had ≥70% improvement and 60% had ≥50% improvement and PIH occurred in 11% of subjects. 28 In this study, the mean beam diameter used was 2.4 mm and the mean fluence was 3.12 J/cm2.
Early in the course of the present study, four subjects developed PIH that followed treatment with larger beam diameters. As the 730 nm picosecond laser had not been previously studied for the treatment of epidermal pigmentation, several spot size and fluence combinations were chosen for the initial treatments to optimize the laser parameters. Treatment with the larger beam diameters of 3–5 mm did not induce the endpoint of immediate skin whitening, but rather only erythema after stacking of laser pulses. It was in these individuals that we observed PIH in one or two lesions in individuals who each had upwards of 50 lesions treated, and the PIH resolved within 3–4 weeks of the application of a topical corticosteroid cream and hydroquinone. Based on these observations, treatment settings were adjusted to smaller spot sizes and higher fluences (Figure 1A,B) and there were no additional occurrences of PIH. The finding of no significant PIH with the picosecond 730 nm laser in our study suggests that the picosecond 730 nm laser may be a preferred modality for the treatment of solar lentigines in darker phototype skin.
Compared with nanosecond pulses, picosecond lasers, with pulse durations at least 10 times shorter than nanosecond‐domain lasers, destroy melanosomes via a largely photoacoustic rather than photothermal effect, and there is better confinement of thermal energy. Light microscopy studies by Negishi et al. 26 show that application of a nanosecond frequency‐doubled Nd:YAG laser to a lentigo produced suprabasal vacuolar change with partial separation of the epidermis and dermis, whereas the picosecond 532 nm laser produced smaller vacuoles and no epidermal separation. Our histologic evaluation produced similar results with larger vacuoles and clefting observed with the nanosecond 532 nm and nanosecond 755 nm lasers and smaller vacuoles without epidermal separation in the specimens that were treated with the picosecond 532 and 730 nm lasers. Scanning electron microscopy (SEM) performed by the same authors showed that the nanosecond 532 nm laser destroyed melanosomes and surrounding cytosol, which appears as clustered material, but no cytosol damage was evident with picosecond 532 nm treatment. In the SEM images, nanosecond laser treatment produced separation of the hemidesmosomes and lamina densa, but this was not observed following picosecond laser treatment.
In our histology studies, hemorrhage was marked in the lesions treated with the nanosecond 532 nm laser (Figure 6D) with rare extravasation of red cells with the picosecond 532 nm laser. Clinically, we observed purpura develop immediately after nanosecond 532 nm treatment and rare petechiae after treatment with the picosecond 532 nm laser. Purpura and petechiae were not observed after treatment of lentigines with the 730 nm picosecond laser, and healing times were reduced compared with historical controls.
In conclusion, this study demonstrated safe and effective treatments using a new 730 nm picosecond‐domain laser for the clearance of lentigines. The results showed good clearance with no adverse events and good subject satisfaction. It should be noted that the majority of subjects in this study had skin type II and III. Additional studies are needed to assess the safety and efficacy of the 730 nm picosecond laser for the treatment of lentigines in darker skin type and other pigmented lesions in general.
ACKNOWLEDGMENTS
The authors would like to thank Jaclyn Coumou, RN and Hannah Cook, RN for their invaluable assistance with this study. The authors acknowledge Doran Rozen and Asaf Mader for critical review of the manuscript. This study was funded by Candela Medical.
Kauvar ANB, Sun R, Bhawan J, Singh G, Ugonabo N, Feng H, et al. Treatment of facial and non‐facial lentigines with a 730 nm picosecond titanium: sapphire laser is safe and effective. Lasers Surg Med. 2022;54:89–97. 10.1002/lsm.23450
REFERENCES
- 1. Ortonne JP, Pandya AG, Lui H, Hexsel D. Treatment of solar lentigines. J Am Acad Dermatol. 2006;54(5 suppl 2):S262–71. [DOI] [PubMed] [Google Scholar]
- 2. Anderson RR, Margolis RJ, Watenabe S, Flotte T, Hruza GJ, Dover JS. Selective photothermolysis of cutaneous pigmentation by Q‐switched Nd:YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989;93(1):28–32. [DOI] [PubMed] [Google Scholar]
- 3. Hruza GJ, Dover JS, Flotte TJ, Goetschkes M, Watanabe S, Anderson RR. Q‐switched ruby laser irradiation of normal human skin. Histologic and ultrastructural findings. Arch Dermatol. 1991;127(12):1799–805. [PubMed] [Google Scholar]
- 4. Negishi K, Akita H, Tanaka S, Yokoyama Y, Wakamatsu S, Matsunaga K. Comparative study of treatment efficacy and the incidence of post‐inflammatory hyperpigmentation with different degrees of irradiation using two different quality‐switched lasers for removing solar lentigines on Asian skin. J Eur Acad Dermatol Venereol. 2013;27(3):307–12. [DOI] [PubMed] [Google Scholar]
- 5. Kang HJ, Na JI, Lee JH, Roh MR, Ko JY, Chang SE. Postinflammatory hyperpigmentation associated with treatment of solar lentigines using a Q‐switched 532‐nm Nd:YAG laser: a multicenter survey. J Dermatolog Treat. 2017;28(5):447–51. [DOI] [PubMed] [Google Scholar]
- 6. Wang CC, Sue YM, Yang CH, Chen CK. A comparison of Q‐switched alexandrite laser and intense pulsed light for the treatment of freckles and lentigines in Asian persons: a randomized, physician‐blinded, split‐face comparative trial. J Am Acad Dermatol. 2006;54(5):804–10. [DOI] [PubMed] [Google Scholar]
- 7. Ho SG, Chan NP, Yeung CK, Shek SY, Kono T, Chan HH. A retrospective analysis of the management of freckles and lentigines using four different pigment lasers on Asian skin. J Cosmet Laser Ther. 2012;14(2):74–80. [DOI] [PubMed] [Google Scholar]
- 8. Kono T, Sakurai H, Groff WF, Chan HH, Takeuchi M, Yamaki T, et al. Comparison study of a traditional pulsed dye laser versus a long‐pulsed dye laser in the treatment of early childhood hemangiomas. Lasers Surg Med. 2005;38:112–115. [DOI] [PubMed] [Google Scholar]
- 9. Chan JC, Shek SY, Kono T, Yeung CK, Chan HH. A retrospective analysis on the management of pigmented lesions using a picosecond 755‐nm alexandrite laser in Asians. Lasers Surg Med. 2016;48(1):23–9. [DOI] [PubMed] [Google Scholar]
- 10. Ross V, Naseef G, Lin G, Kelly M, Michaud N, Flotte TJ, et al. Comparison of responses of tattoos to picosecond and nanosecond Q‐switched neodymium:YAG lasers. Arch Dermatol. 1998;134(2):167–71. [DOI] [PubMed] [Google Scholar]
- 11. Izikson L, Farinelli W, Sakamoto F, Tannous Z, Anderson RR. Safety and effectiveness of black tattoo clearance in a pig model after a single treatment with a novel 758 nm 500 picosecond laser: a pilot study. Lasers Surg Med. 2010;42:640–46. [DOI] [PubMed] [Google Scholar]
- 12. Kauvar ANB, Keaney TC, Alster T. Laser treatment of professional tattoos with a 1064/532‐nm dual‐wavelength picosecond laser. Dermatol Surg. 2017;43(12):1434–40. [DOI] [PubMed] [Google Scholar]
- 13. Bernstein EF, Schomacker KT, Basilavecchio LD, Plugis JM, Bhawalkar JD. A novel dual‐wavelength, Nd:YAG, picosecond‐domain laser safely and effectively removes multicolor tattoos. Lasers Surg Med. 2015;47(7):542–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herd RM, Alora MB, Smoller B, Arndt KA, Dover JS. A clinical and histologic prospective controlled comparative study of the picosecond titanium:sapphire (795 nm) laser versus the Q‐switched alexandrite (752 nm) laser for removing tattoo pigment. J Am Acad Dermatol. 1999;40:603–6. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein EF, Schomacker KT, Basilavecchio LD, Plugis JM, Bhawalkar JD. Treatment of acne scarring with a novel fractionated, dual‐wavelength, picosecond‐domain laser incorporating a novel holographic beam‐splitter. Lasers Surg Med. 2017;49(9):796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernstein EF. News, views, and reviews: a tool for my laser practice I simply can't do without: shining a light on my favorite light (source). J Drugs Dermatol. 2017;16(9):939–44. [PubMed] [Google Scholar]
- 17. Kung KY, Shek SY, Yeung CK, Chan HH. Evaluation of the safety and efficacy of the dual wavelength picosecond laser for the treatment of benign pigmented lesions in Asians. Lasers Surg Med. 2019;51(1):14–22. [DOI] [PubMed] [Google Scholar]
- 18. Wat H, Yee‐nam Shek S, Yeung CK, Chan HH. Efficacy and safety of picosecond 755‐nm alexandrite laser with diffractive lens array for non‐ablative rejuvenation in Chinese skin. Lasers Surg Med. 2019;51:8–13. [DOI] [PubMed] [Google Scholar]
- 19. Tanghetti EA, Hoffmann KA, Hoffmann K. Short‐pulsed laser for the treatment of tattoos, pigmented lesions, scars and rejuvenation. Semin Cutan Med Surg. 2017;36:148–54. [DOI] [PubMed] [Google Scholar]
- 20. Wu DC, Goldman MP. Successful treatment of chronic venous stasis hyperpigmentation of the lower limbs with the picosecond alexandrite laser. Dermatol Surg. 2018;44(6):881–883. [DOI] [PubMed] [Google Scholar]
- 21. Wong THS. Picosecond laser treatment for acquired bilateral nevus of Ota‐like macules. JAMA Dermatol. 2018;154(10):1226–28. [DOI] [PubMed] [Google Scholar]
- 22. Bernstein EF, Schomacker KT, Shang X, Alessa D, Algzlan H, Paranjape A. The first commercial 730 nm picosecond‐domain laser is safe and effective for treating multicolor tattoos. Lasers Surg Med. 2020;53:89–94. 10.1002/lsm.23237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alegre‐Sanchez A, Jiménez‐Gómez N, Moreno‐Arrones ÓM, Fonda‐Pascual P, Pérez‐García B, Jaén‐Olasolo P, et al. Treatment of flat and elevated pigmented disorders with a 755‐nm alexandrite picosecond laser: clinical and histological evaluation. Lasers Med Sci. 2018;33(8):1827–31. [DOI] [PubMed] [Google Scholar]
- 24. Vachiramon V, Iamsumang W, Triyangkulsri K. Q‐switched double frequency Nd:YAG 532‐nm nanosecond laser vs. double frequency Nd:YAG 532‐nm picosecond laser for the treatment of solar lentigines in Asians. Lasers Med Sci. 2018;33(9):1941–47. [DOI] [PubMed] [Google Scholar]
- 25. Chan MWM, Shek SY, Yeung CK, Chan HH. A prospective study in the treatment of lentigines in asian skin using 532 nm picosecond Nd:YAG laser. Lasers Surg Med. 2019;51(9):767–73. [DOI] [PubMed] [Google Scholar]
- 26. Negishi K, Akita H, Matsunaga Y. Prospective study of removing solar lentigines in Asians using a novel dual‐wavelength and dual‐pulse width picosecond laser. Lasers Surg Med. 2018;50:851–58. [DOI] [PubMed] [Google Scholar]
- 27. Lee SJ, Han HS, Hong JK, Park KY, Seo SJ. Successful treatment of pigmentary disorders in Asians with a novel 730‐nm picosecond laser. Lasers Surg Med. 2020;52:923–27. [DOI] [PubMed] [Google Scholar]
- 28. Lipp MB, Angra K, Wu DC. Safety and efficacy of a novel 730 nm picosecond titanium sapphire laser for the treatment of benign pigmented lesions. Lasers Surg Med. 2020;53:429–34. [DOI] [PubMed] [Google Scholar]


