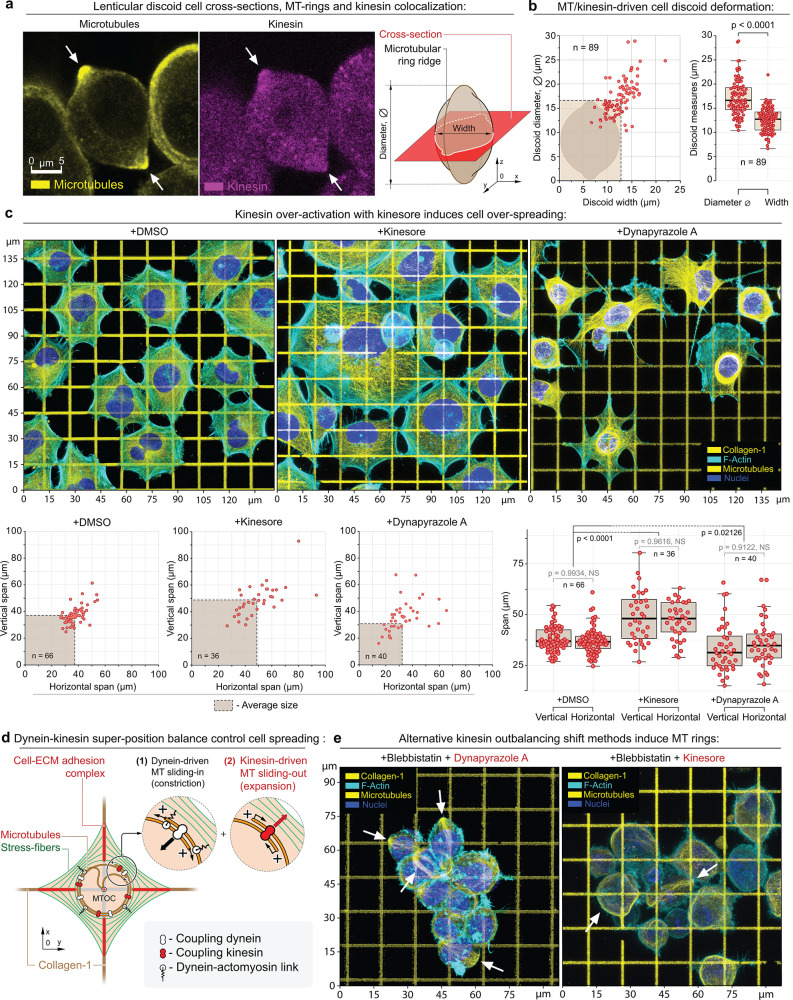
Figure 3.
Structural analysis of the MDA-MB-231 cells during alternative dynein–kinesin balance shift mechanisms. (a) MT ring median-plane cross-section in MDA-MB-231 cells (left) as shown in the schematic (right) in +Dynapyrazole A+Blebbistatin. Note kinesin accumulation at the MT rings (arrows); see also Figure SI3a–d. (b) MT rings expand and deform cells into the lenticular discoids (MT ring diameter > cell width). The plots depict the measurements of the cell discoid diameters (diameter of the MT ring) and the cells’ widths (transverse cell dimension). The dashed box depicts the averaged cell discoid diameter and width boundaries (means). (c) Top row – Adhesion and spreading of MDA-MB-231 cells on the collagen-1 grids (G′ = 50 kPa) in control condition (+DMSO) and during kinesin overactivation (+Kinesore), compared to the direct dynein inactivation (+Dynapyrazole A). Kinesin overactivation with +Kinesore does not inhibit the dynein activity, yet induces a significant increase of the biaxial spreading of cells in comparison to the control cells. Alternatively, dynein–kinesin balance shift toward the kinesin activity via the direct dynein inhibition (+Dynapyrazole A) induces a visible reduction of cell spreading and “dendritization” of cells that is similar to the loss of cell contractility shape during Blebbistatin-induced myosin II inhibition (Figure 1, +Blebbistatin). The loss of cell contractility in Dynapyrazole A is related to two main factors:74 the loss of MT-to-actomyosin mechanical cross-linking and force transmission as well as the loss of the dynein-driven mechanical contractility within the MT network (+Dynapyrazole A). Bottom row – Morphometric analysis of control (+DMSO) and +Kinesore- and +Dynapyrazole A-treated cells indicate a significant increase in cell biaxial spreading, driven by kinesin-mediated MT network expansion. (d) Suggested mechanism for the MT–actomyosin interaction in cells on the collagen grids. Dyneins and kinesins control the (1) MT network compaction and (2) MT network expansion, respectively. Dyneins also cross-link the MT network to the actomyosin cytoskeleton (stress-fibers), i.e., actomyosin, adhesion-bound matrix, adhesion complex, MTs, and MT motor protein subsystems are united into a single mechanical system. See also Figure SI4a. (e) Alternative methods for outbalancing the kinesin activity via +Dynapyrazole A+Blebbistatin and +Kinesore+Blebbistatin induce MT rings (arrows) and cell discoids. F-actin is labeled with Phalloidin-ATTO 647N. Chromatin is labeled with Hoechst. Pairwise one-way t test-derived p values are shown on the plots with corresponding n (size of individual cells measurement sets) for each condition, generated in triplicates.