Figure 8.
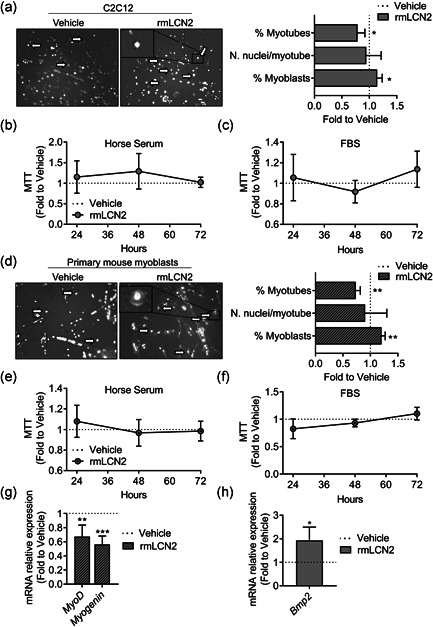
Effect of rmLCN2 on muscle cells in vitro. (a) C2C12 cells were treated with 1 µg/ml LCN2 under a myogenic differentiation medium (DMEM supplemented with 5% horse serum) for 4 days. Then cells were fixed and stained with DAPI to evidence nuclei. Merged phase‐contrast DAPI images were collected to analyse the % of myonuclei, the % of myoblasts and the number of nuclei/myotube. (b) C2C12 were treated with 1 µg/ml rmLCN2 for 24, 48, or 72 h under differentiation medium or (c) DMEM plus 10% FBS. MTT was carried out to analyse cell metabolic activity. (d) Primary CD1 mouse myoblasts were treated with 200 ng/ml rmLCN2 for 24, 48, or 72 h under differentiation medium, then cells were fixed and stained with DAPI to evidence nuclei. Merged phase‐contrast DAPI images were collected to analyse the % of myonuclei, the % of myoblasts and the number of nuclei/myotube. (e) Myoblasts were treated with 200 ng/ml rmLCN2 for 24, 48, or 72 h under differentiation medium or (f) DMEM/F10 plus 20% FBS and 2.5 ng/ml bFGF. MTT was carried out to analyse cell metabolic activity. (g) Myoblasts were treated for 4 days with 200 ng/ml rmLCN2 under differentiation medium, then RNA was extracted, reverse‐transcribed, and Myod and Myogenin expression was analysed. (h) C2C12 cells were treated as described in (a), then RNA was extracted, reverse‐transcribed and subjected to signal transduction pathway finder RT2‐array. After applying cut‐off criteria (threshold cycle [C t]<30, p‐value <.05) and validating the results with a separate set of primer, we found Bmp2 to be significantly upregulated in cells treated with rmLCN2. (a, d) Arrow and insets: myoblast‐like cells. Student's t test. *p < .05; **p < .01 versus vehicle. DMEM, Dulbecco's modified Eagle's medium; bFGF, basic fibroblast growth factor; DAPI, 4′,6‐diamidino‐2‐phenylindole; FBS, fetal bovine serum; MTT, 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium
