Abstract
Objectives
To assess long‐term neurodevelopmental outcomes in children after radiofrequency ablation (RFA) for twin reversed arterial perfusion (TRAP) sequence.
Methods
This cross‐sectional study included children who underwent RFA for the TRAP sequence between 2012 and 2018. We assessed neurodevelopment in children using the Kinder Infant Development Scale, a validated questionnaire. The developmental quotient (DQ) assessed in nine subscales was calculated as the developmental age divided by the chronological age. Neurodevelopmental delay (NDD) was defined as a DQ of <70 points.
Results
In total, 38 children from 37 pregnancies underwent RFA for the TRAP sequence during the study period; 6 fetuses died in utero. We sent the questionnaire to the parents of the 32 surviving children and obtained answers for 27 (84%). The median age at the assessment was 2 years and 5 months old. The median total DQ was 111 (80–150). Most median DQs in the nine subscales were above 70. The incidence of NDD was 0% (0/27). There were no marked differences in DQ by chorionicity.
Conclusions
Children who survived after RFA for TRAP sequence showed favorable long‐term neurodevelopmental outcomes. Radiofrequency ablation seems to rarely affect fetal neurodevelopment. Pregnant women with TRAP sequence are encouraged to be treated by RFA.
Key points
What's already known about this topic?
Radiofrequency ablation (RFA), which induces the coagulation of tissue, is commonly used to treat twin reversed arterial perfusion (TRAP) sequence because of the minimal invasiveness of the technique.
The survival rate of the pump twin of TRAP sequence treated by RFA is more than 80%; however, the long‐term neurodevelopmental outcomes of survivors have rarely been investigated.
What does this study add?
Pump twins of TRAP sequence who survived after RFA showed favorable long‐term neurodevelopmental outcomes with a median developmental quotient of more than 100.
Radiofrequency ablation in fetal surgery itself seems to rarely affect fetal neurodevelopment.
1. INTRODUCTION
Twin reversed arterial perfusion (TRAP) sequence is a complicated twin pregnancy, accounting for 1% of monochorionic twin pregnancies. The acardiac twin has no functional heart and receives a perfused blood flow from the pump twin. The pump twin has a risk of high‐output cardiac failure, which can lead to hydrops fetalis and/or sudden fetal death in utero when the blood perfusion to the acardiac twin continues. 1 The survival outcome of pump twin has been improved by fetal surgeries that interrupt the perfusion to the acardiac twin, including radiofrequency ablation (RFA), fetoscopic cord ligation, cord coagulation by laser, monopolar or bipolar cord coagulation, fetoscopic laser coagulation of placental anastomoses and intrafetal laser ablation. 2 , 3 , 4
Radiofrequency ablation, which induces coagulation of tissue, has been applied to treat TRAP sequence since 2000s 5 Since then, RFA has been commonly used because of the minimal invasiveness of this technique. The survival rate of the pump twin after RFA is reportedly 85%–88%, and the preterm birth rate before 32 or 34 weeks' gestation is reportedly 9%–23%. 6 , 7 These results led to the coverage of the cost of the RFA procedure by Japan National Health Insurance in 2019. Radiofrequency ablation is now the third‐most frequently performed fetal therapy in Japan, following fetoscopic laser surgery (FLS) for twin‐to‐twin transfusion syndrome (TTTS) and thoracoamniotic shunting for fetal hydrothorax. 8
The neurodevelopmental outcome is a major point of interest for fetal therapy, as the fetal period is crucial for neurodevelopment. Regarding treatment of TTTS, our concern is shifting from the survival outcome to the neurodevelopmental outcome of children after FLS. There have been some reports of long‐term neurodevelopment assessments of children who underwent FLS for TTTS. 9 , 10 , 11 However, the neurodevelopmental outcome of the pump twin of TRAP sequence after fetal intervention has rarely been investigated.
The present study assessed the neurodevelopmental outcome of the pump twin of TRAP sequence after RFA.
2. MATERIALS AND METHODS
2.1. Design and study protocol
This was a cross‐sectional study for children who underwent RFA for TRAP sequence at our hospital between January 2012 and June 2018. We sent a validated questionnaire to the parents of those children for a neurodevelopmental evaluation in February 2019. The questionnaires that the parents filled out and returned to us between March 2019 and May 2019 were then assessed.
Consent for this research was obtained from the parents. The protocol was approved by the ethics committee of the National Center for Child Health and Development (No. 2053).
2.2. Procedure and management
Twin reversed arterial perfusion sequence was defined as a monochorionic pregnancy with the acardiac mass perfused by a pump twin confirmed by detecting the reversed arterial flow in the cord of the acardiac twin. Radiofrequency ablation was offered when a significant risk of death of the pump twin was suspected, as indicated by polyhydramnios (maximum vertical pocket [MVP] ≥8 cm at any gestational week), cardiac strain in the pump twin (pulsatile flow in the umbilical vein and/or reverse flow in the ductus venosus) or a large acardiac mass (abdominal circumference [AC] of the acardiac twin larger than that of the pump twin). All procedures were performed between 15 and 26 weeks of gestation.
Radiofrequency ablation was performed using expandable tines though a multistep coagulation method described previously. 6 We used the RF3000™ Radiofrequency Ablation System (Boston Scientific) with LeVeen™ SuperSlim Needle Electrodes (Boston Scientific), which consist of a 17‐gauge cannula and a 15‐cm‐long and 20‐mm‐wide expandable umbrella with 8 tines. All procedures were performed percutaneously with ultrasound guidance. Five operators were involved, and one of the senior doctors (HS) attended all cases.
Under locoregional anesthesia for the mother and transplacental sedation for the fetus, we inserted the cannula into the pelvis near the umbilical cord insertion of the acardiac twin. Postoperative management, including ultrasound examinations, were performed at our center for one week. Most pregnant women then returned to the referring perinatal centers, and prenatal and postnatal management were performed at these centers, which were part of the national neonatal network in Japan. A brain ultrasound examination of the newborn was performed when a newborn was premature or abnormalities were suspected. Brain magnetic resonance imaging (MRI) was performed when the clinical examination or ultrasonography suggested any neurological abnormality in the newborn or infant. The perinatal outcome was reported to our center from each center after discharge of the mother and infant.
2.3. Neurodevelopmental testing
We assessed the neurodevelopment of the children using the Kinder Infant Development Scale (KIDS), a questionnaire answered by their parents, which was standardized in 1988–1989 using 6000 children aged 0–6 years old by the Center of Developmental Education and Research in Japan. This test has good correlation with the Kyoto Scale of Psychological Development 2001, 12 an assessment reported to be comparable to the Bayley Scales of Infant Development, second and third editions. 13 , 14 , 15 This test has been validated with the family‐rated Ages & Stages Questionnaires, Third Edition, family‐rated Ability for Basic Movement Scale for Children and Ability for Basic Movement Scale for Children type T and staff‐rated Functional Independence Measure for Children (WeeFIM). 16 The KIDS consists of nine subscales for behavior: physical motor, manipulation, receptive language, expressive language, language concepts, social relationships with children, social relationships with adults, discipline and feeding. In each subscale, behaviors that can be performed by the child are checked. Three different questionnaires of the KIDS were used, depending on the age of the children (under 1 year old, 1–2 years old and 3–6 years old). Using the nine areas of development, this test produces a clear profile of the developmental age and developmental quotient (DQ) of the child. The DQ is calculated as the developmental age divided by the chronological age and is adjusted by the gestational age (GA) at birth in children under 36 months old.
2.4. Outcome variables and statistical methods
The primary outcome was the incidence of neurodevelopmental delay (NDD). Neurodevelopmental delay was defined as a DQ < 70 points. 14 The secondary outcome was the DQ in each subscale of the KIDS. Language concepts, social relationships with children and discipline were assessed in children ≥1 year old. Feeding was assessed in children ≤2 years old. Prenatal parameters, including chorionicity (monochorionic diamniotic [MCDA], monochorionic monoamniotic [MCMA] or monochorionic triamniotic [MCTA]), preoperative fetal condition (AC ratio of acardiac twin to pump twin, cardiac overload and MVP before RFA), GA at RFA and preterm premature rupture of membrane (PPROM) before 34 weeks of gestation, were collected. Cardiac overload was defined as a pump twin with at least one of the following conditions: cardiomegaly, mitral or tricuspid valve regurgitation, venous Doppler abnormality (pulsatile flow in the umbilical vein and/or reverse flow in the ductus venosus), hydrops or combined cardiac output (CCO) >600 ml/min/kg that is above 90th percentile of normal range. 17 The cardiac output was calculated as follows: (valve diameter [cm]/2)2 × 3.14 × velocity time integral (cm) × heart rate (beat/min). The CCO was the sum of the cardiac output from both ventricles. Postnatal parameters, including the GA at delivery, birth weight, sex, associated anomaly and major neurological complications in the neonatal period, were also collected. Major neurological complications were defined as grade 3 or 4 intraventricular hemorrhage (IVH), periventricular leukomalacia or bilateral hearing loss screened by otoacoustic emission or auditory brainstem response.
Descriptive statistics were conducted using the SPSS Statistics version 26 software program (IBM Corp.). The data were described by number, average ± standard deviation or median (range).
3. RESULTS
In total, 38 fetuses from 37 pregnancies underwent RFA for the TRAP sequence during the study period. Six fetuses died in utero after RFA, and no deaths occurred after birth. The 32 surviving children (ranging in age from 4 months to 6 years old) were included (Figure 1). We sent the questionnaire to the parents of the 32 children and obtained answers for 27 (84%), including 23 MCDA twins, 2 MCMA twins and 2 MCTA triplets.
FIGURE 1.
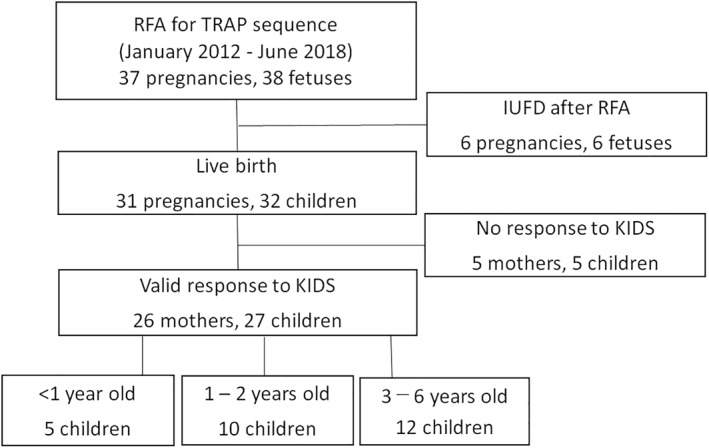
Flowchart of the study. IUFD, intrauterine fetal death; KIDS, Kinder Infant Development Scale; RFA, radiofrequency ablation; TRAP, twin reversed arterial perfusion
The perinatal characteristics of the children are shown in Table 1. The children were classified into three groups by their age: <1 year old, 1–2 years old and 3–6 years old. The GA at RFA was 21.1 (16.4–25.7) weeks. The average AC ratio of the acardiac twin to the pump twin was 1.35 ± 0.30, and 13 children (48%) showed cardiac overload in utero before RFA. Preterm premature rupture of membrane before 34 weeks of gestation occurred in three pregnancies (11%) after RFA. The median GA at birth was 38.4 (24.6–41.1) weeks. Nine cases (33%) had preterm birth. The average and standard deviation of the birth weight was 2574 ± 761 g. Congenital anomaly was found in three children, including two cardiac disease and one kidney disease, although major neurological complications were not found.
TABLE 1.
Perinatal characteristics of the assessed children
| <1 year old | 1–2 years old | 3–6 years old | Total | |
|---|---|---|---|---|
| Case number | 5 | 10 | 12 | 27 |
| Age (years–months) | 0y9m (0y4m, 0y10m) | 1y10m (1y1m, 2y8m) | 5y6m (3y4m, 6y9m) | 2y5m (0y4m, 6y9m) |
| Male | 2 (40) | 5 (50) | 5 (42) | 12 (44) |
| Chorionicity | ||||
| MCDA | 4 | 8 | 11 | 23 |
| MCMA | 1 | 0 | 1 | 2 |
| MCTA | 0 | 2 | 0 | 2 |
| AC ratio | 1.41 ± 0.31 | 1.27 ± 0.31 | 1.39 ± 0.29 | 1.35 ± 0.30 |
| Cardiac overload | 0 (0) | 4 (40) | 9 (75) | 13 (48) |
| MVP (mm) | 61 ± 17 | 60 ± 19 | 71 ± 18 | 65 ± 18 |
| GA at RFA (weeks) | 18.7 (16.4, 25.7) | 21.4 (16.4, 24.7) | 21.3 (16.4, 25.0) | 21.1 (16.4, 25.7) |
| PPROM <34 weeks | 2 (40) | 0 (0) | 1 (8) | 3 (11) |
| GA at delivery (weeks) | 36.4 (30.7, 39.9) | 39.4 (31.4, 41.1) | 38.4 (24.6, 40.9) | 38.4 (24.6, 41.1) |
| Preterm birth | 3 (60) | 3 (30) | 3 (25) | 9 (33) |
| Birth weight (g) | 2327 ± 676 | 2580 ± 892 | 2645 ± 761 | 2574 ± 761 |
| Major neurological complication | 0 | 0 | 0 | 0 |
| Associated anomaly | 0 (0) | 1 (10) | 2 (17) | 3 (11) |
| DORV | VSD | DORV | ||
| HSK | VSD, | |||
| HSK |
Note: Data are shown as the number (%), average ± standard deviation or median (minimum, maximum).
Abbreviations: AC, abdominal circumference; DORV, double‐outlet right ventricle; GA, gestational age; HSK, horseshoe kidney; MCDA, monochorionic diamniotic; MCMA, monochorionic monoamniotic; MCTA, monochorionic triamniotic; MVP, maximum vertical pocket; PPROM, preterm premature rupture of membranes; RFA, radiofrequency ablation; VSD, ventricular septal defect.
Table 2 shows the total DQ and each DQ of the nine subscales of the children after RFA assessed by the KIDS. The median age at the assessment was 2 years and 5 months (4 months–6 years and 9 months) old. The median total DQ was 111 (80–150). Almost all of the median DQs in the nine subscales were above 70. Only one boy who was born at 32 weeks of gestation and assessed at 7 months old showed a low DQ of 20 in the physical motor subscale; however, he showed a total DQ of 80. On follow‐up evaluations, he started to walk with no signs of cerebral palsy at 1 year and 5 months old. The incidence of NDD was 0% (0/27).
TABLE 2.
Developmental quotient in the children after RFA assessed by the KIDS
| Subscales | <1 year old | 1–2 years old | 3–6 years old | Total |
|---|---|---|---|---|
| Number | 5 | 10 | 12 | 27 |
| Total | 110 (80, 150) | 117 (93, 133) | 109 (94, 133) | 111 (80, 150) |
| Physical motor | 100 (20, 130) | 97 (84, 125) | 96 (70, 113) | 99 (20, 130) |
| Manipulation | 110 (80, 125) | 109 (86, 133) | 116 (88, 150) | 111 (80, 150) |
| Receptive language | 114 (89, 200) | 125 (97, 158) | 104 (90, 151) | 117 (89, 200) |
| Expressive language | 100 (80, 200) | 111 (91, 125) | 109 (84, 145) | 110 (80, 200) |
| Language concepts | ‐ | 132 (103, 285) | 114 (94, 133) | 117 (94, 285) |
| Social relationships with children | ‐ | 121 (84, 158) | 96 (85, 128) | 104 (84, 158) |
| Social relationships with adults | 100 (86, 175) | 120 (79, 150) | 105 (83, 146) | 106 (79, 175) |
| Discipline | ‐ | 130 (72, 142) | 106 (74, 140) | 110 (72, 142) |
| Feeding | 100 (80, 133) | 100 (72, 137) | ‐ | 100 (72, 137) |
Note: Data are shown as the Median (minimum, maximum). Language concepts, social relationships with children and discipline were assessed in children who were ≥1 year old. Feeding was assessed in children who were ≤2 years old. The developmental quotients are adjusted by gestational age at birth in the children <36 months old.
Abbreviations: KIDS, Kinder Infant Development Scale; RFA, radiofrequency ablation.
The perinatal characteristics of the children classified by chorionicity (MCDA, MCMA and MCTA) and their DQs are shown in Table 3. The median total DQs were 111, 103 and 125 for MCDA, MCMA and MCTA, respectively. Furthermore, there was no marked difference in each DQ of the nine subscales by chorionicity.
TABLE 3.
Perinatal characteristics and developmental quotient of the assessed children classified by chorionicity
| Chorionicity | MCDA | MCMA | MCTA |
|---|---|---|---|
| Number | 23 | 2 | 2 |
| Age (years–months) | 2y8m (0y4m, 6y9m) | 3y3m (0y10m, 5y8m) | 1y2m |
| Female | 14 | 1 | 0 |
| GA at RFA (weeks) | 20.3 (16.4, 25.7) | 23.1 (21.1, 25.0) | 21.4 |
| PPROM <34 weeks | 3 | 0 | 0 |
| GA at delivery (weeks) | 38.7 (24.6, 41.1) | 38.3 (37.1, 39.4) | 32.6 |
| Associated anomaly | VSD 1, HSK 1 | ‐ | DORV 1 |
| DQ | |||
| Total | 111 (80, 150) | 103 (96, 110) | 125 (117, 133) |
| Physical motor | 97 (20, 125) | 101 (72, 130) | 113 (100, 125) |
| Manipulation | 114 (80, 150) | 99 (88, 110) | 117 (100, 133) |
| Receptive language | 119 (86, 200) | 101 (100, 103) | 129 (117, 142) |
| Expressive language | 110 (80, 200) | 101 (100, 103) | 125 (125, 125) |
| Language concepts | 116 (94, 285) | 115 | 167 (167, 167) |
| Social relationships with children | 107 (84, 150) | 85 | 129 (100, 158) |
| Social relationships with adults | 106 (79, 175) | 101 (100, 101) | 138 (125, 150) |
| Discipline | 107 (72, 142) | 74 | 133 (133, 133) |
| Feeding | 97 (72, 137) | 110 | 125 (125, 125) |
Note: Data are shown as median (minimum, maximum). Language concepts, social relationships with children and discipline were assessed in children who were ≥1 year old. Feeding was assessed in children who were ≤2 years old. The developmental quotients are adjusted by gestational age at birth in the children <36 months old.
Abbreviations: GA, gestational age; HSK, horseshoe kidney; MCDA, monochorionic diamniotic; MCMA, monochorionic monoamniotic; MCTA, monochorionic triamniotic; PPROM, preterm premature rupture of membranes; RFA, radiofrequency ablation.
4. DISCUSSION
We reported the long‐term neurodevelopmental outcomes in 27 surviving children after RFA for TRAP sequence using a validated questionnaire. The median total DQ was 111 (80–150) at a median age of assessment of 2 years and 5 months (4 months–6 years and 9 months) old. All of the surviving children who underwent RFA for TRAP sequence showed favorable neurological developments, regardless of chorionicity. This is the first report of long‐term neurodevelopmental assessments on pump twins in TRAP sequence treated by RFA.
There are only a few reports of the long‐term neurodevelopmental outcomes in children after fetal surgery for TRAP sequence. Lewi et al. reported the developmental outcome of 22 children who underwent cord coagulation for TRAP sequence, and 3 of them had developmental delay. 18 The GA at delivery of those three children was 28–29 weeks. van Klink et al. reported the long‐term neurodevelopmental outcome of 74 children who underwent selective feticide using various methods, including fetoscopic laser cord coagulation, bipolar cord coagulation, interstitial laser coagulation and RFA. Among the 74 cases, 19 were TRAP sequence, in which 1 child who underwent interstitial laser coagulation was born at 41 weeks of gestation and had cerebral palsy and cognitive impairment. 19 In contrast, our results showed that all 27 children who survived after RFA for TRAP sequence had favorable neurodevelopment outcomes. Although one 7‐month‐old boy born preterm showed a low DQ in the physical motor subscale with a normal total DQ, he caught up with regard to his physical motor function later.
In this study, the median GA at delivery after RFA was 38 weeks, and only 1 child was born before 28 weeks of gestation. Preterm premature rupture of membrane less than 34 weeks of gestation occurred in 3 of 26 pregnancies (11.5%). We previously reported that the survival rate of the pump twin after RFA was 85% in 40 cases, and the median GA at delivery was 37 weeks with an 8.6% preterm birth rate before 34 weeks of gestation. 6 A systematic review of six reports published in 2014 found that the survival rate of children with TRAP sequence after RFA was 81.5% (88/108). 20 However, those 6 reports all involved under 10 cases each, except for 1 report by Lee et al. in 2013 that included 98 cases with a median GA at delivery of 37 weeks and 17% PPROM rate. 3 Therefore, the preterm birth rate was low in TRAP sequence cases treated by RFA. A low GA at birth is considered a strong risk factor for neurodevelopment impairment in TTTS survivors after FLS. 9 The low rate of premature delivery after RFA might contribute to favorable long‐term neurodevelopmental outcomes.
Radiofrequency ablation is also used to perform selective reduction in monochorionic twin pregnancies. There are a few reports of the long‐term neurodevelopmental outcome in children using RFA for selective reduction. 21 In a report assessing the long‐term neurodevelopmental outcome in children after selective reduction in monochorionic twin pregnancies, 5 of 74 children underwent RFA and showed no neurodevelopmental impairments. 19 Among 131 children who underwent selective reduction, 11 underwent RFA, and 1 had grade Ⅳ IVH. 22 Another study reported abnormal brain MRI findings in 3/100 children who underwent selective reduction of co‐twin by RFA. 23 A favorable long‐term neurodevelopmental outcome seems to be expected in children who undergo RFA for complicated monochorionic twins. A vaporing appearance caused by tissue coagulation which is induced by RFA is often observed during fetal surgery. The appearance of such vapors is worrisome since it may have some adverse effects on fetal neurology. However, these present and previous findings suggest that RFA in fetal surgery seems to rarely affect the fetal neurodevelopment.
We successfully assessed the neurodevelopmental outcome after fetal therapy using the KIDS, even though the patients lived in an area that was far from our hospital. We obtained answers for 84% of the children. Long‐term neurodevelopmental outcomes of children were assessed using the KIDS to evaluate the influence of reproductive technology on treated children. 24 , 25 A study in France used the Ages and Stages Questionnaires (ASQ) to assess the NDD in children with TTTS. 26 The ASQ is a questionnaire answered by parents and a validated screening tool for abnormal development of children, similar to the KIDS. Although those studies were performed before the COVID‐19 pandemic, the KIDS and ASQ are commonly used to reduce human contact during developmental assessments.
Long‐term neurodevelopment of children after fetal therapy for complicated twin pregnancies has been mainly reported in TTTS. The incidence of NDD in 188 children at 3 years old following FLS for TTTS in our hospital was 8.5%. 9 The average GA at birth in the children was 33 weeks of gestation. The incidence of NDD in children after FLS for TTTS was about 10% in several reports from other countries. 10 , 11 The incidence of NDD in children after FLS in TTTS in previous reports was higher than that after RFA in TRAP sequence. The average GA at birth in the children after FLS for TTTS in previous reports was about 33 weeks, which is lower than that after RFA for TRAP sequence. Furthermore, the direction of abnormal blood flow in TRAP sequence is constant from a pump twin to an acardiac twin; however, in TTTS, the direction of abnormal blood flow between the donor and recipient is complicated. The differences in the GA at birth and the pathophysiology between TRAP sequence and TTTS might have resulted in the difference in the long‐term neurodevelopmental outcome.
Several limitations associated with the present study warrant mention. First, we were unable to assess the neurodevelopment in five children (16%) after RFA because responses to the KIDS were not returned. This is a major limitation of questionnaire‐based assessments. Second, the ages of the children at the assessment widely ranged from 4 months to 6 years and 9 months old because of the cross‐sectional nature of the study. Only 44% (12/27) of cases were over 3 years old.
In conclusion, children who survived after RFA for TRAP sequence showed favorable long‐term neurodevelopmental outcomes. Radiofrequency ablation seems to rarely affect the fetal neurodevelopment. These results encourage pregnant women suffering from TRAP sequence to be treated by RFA.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee at the National Center for Child Health and Development (No. 2053).
ACKNOWLEDGMENTS
We thank the doctors who referred the TRAP sequence patients to us for RFA and cared for these patients after RFA at their perinatal centers. This work was funded by the Grant of National Center for Child Health and Development 2020 B‐6 of Japan.
Ozawa K, Wada S, Muromoto J, et al. Long‐term neurodevelopmental outcomes of the pump twin in twin reversed arterial perfusion sequence treated by radiofrequency ablation. Prenat Diagn. 2021;41(12):1575‐1581. 10.1002/pd.6048
DATA AVAILABILITY STATEMENT
Research data are not shared yet due to the ongoing nature of the research project.
REFERENCES
- 1. Moore TR, Gale S, Benirschke K. Perinatal outcome of forty‐nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907‐912. [DOI] [PubMed] [Google Scholar]
- 2. Hecher K, Lewi L, Gratacos E, Huber A, Ville Y, Deprest J. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol. 2006;28:688‐691. [DOI] [PubMed] [Google Scholar]
- 3. Lee H, Bebbington M, Crombleholme TM, North American Fetal Therapy Network . The North American Fetal Therapy Network Registry data on outcomes of radiofrequency ablation for twin‐reversed arterial perfusion sequence. Fetal Diagn Ther. 2013;33:224‐229. [DOI] [PubMed] [Google Scholar]
- 4. Tavares de Sousa M, Glosemeyer P, Diemert A, Bamberg C, Hecher K. First‐trimester intervention in twin reversed arterial perfusion sequence. Ultrasound Obstet Gynecol. 2020;55:47‐49. [DOI] [PubMed] [Google Scholar]
- 5. Tsao K, Feldstein VA, Albanese CT, et al. Selective reduction of acardiac twin by radiofrequency ablation. Am J Obstet Gynecol. 2002;187:635‐640. [DOI] [PubMed] [Google Scholar]
- 6. Sugibayashi R, Ozawa K, Sumie M, Wada S, Ito Y, Sago H. Forty cases of twin reversed arterial perfusion sequence treated with radio frequency ablation using the multistep coagulation method: a single‐center experience. Prenat Diagn. 2016;36:437‐443. [DOI] [PubMed] [Google Scholar]
- 7. Wagata M, Murakoshi T, Ishii K, Muromoto J, Sasahara J, Murotsuki J. Radiofrequency ablation with an internally cooled electrode for twin reversed arterial perfusion sequence. Fetal Diagn Ther. 2016;40:110‐115. [DOI] [PubMed] [Google Scholar]
- 8. Sago H, Wada S. Fetal therapies as standard prenatal care in Japan. Obstet Gynecol Sci. 2020;63:108‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsushima S, Ozawa K, Sugibayashi R, et al. Neurodevelopmental impairment at 3 years of age after fetoscopic laser surgery for twin‐to‐twin transfusion syndrome. Prenat Diagn. 2020;40:1013‐1019. [DOI] [PubMed] [Google Scholar]
- 10. Gray PH, Poulsen L, Gilshenan K, Soong B, Cincotta RB, Gardener G. Neurodevelopmental outcome and risk factors for disability for twin‐twin transfusion syndrome treated with laser surgery. Am J Obstet Gynecol. 2011;204:159.e151‐156. [DOI] [PubMed] [Google Scholar]
- 11. Van Klink JM, Koopman HM, Rijken M, Middeldorp JM, Oepkes D, Lopriore E. Long‐term neurodevelopmental outcome in survivors of twin‐to‐twin transfusion syndrome. Twin Res Hum Genet. 2016;19:255‐261. [DOI] [PubMed] [Google Scholar]
- 12. Aoki S, Hashimoto K, Ikeda N, et al. Comparison of the Kyoto Scale of Psychological Development 2001 with the parent‐rated Kinder Infant Development Scale (KIDS). Brain Dev. 2016;38:481‐490. [DOI] [PubMed] [Google Scholar]
- 13. Matsuzaki T, Matsui M, Ichida F, et al. Neurodevelopment in 1‐year‐old Japanese infants after congenital heart surgery. Pediatr Int. 2010;52:420‐427. [DOI] [PubMed] [Google Scholar]
- 14. Tatsuta N, Suzuki K, Sugawara T, Nakai K, Hosokawa T, Satoh H. Comparison of Kyoto scale of psychological development and Bayley scales of infant development second edition among Japanese infants. J Spec Educ Res. 2013;2:17‐24. [Google Scholar]
- 15. Kono Y, Yonemoto N, Kusuda S, et al. Developmental assessment of VLBW infants at 18 months of age: a comparison study between KSPD and Bayley III. Brain Dev. 2016;38:377‐385. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto K, Sakamoto N, Takekoh M, et al. Validity of the family‐rated Kinder Infant Development Scale (KIDS) for children. Pediatr Ther. 2013;03:153. [Google Scholar]
- 17. Kiserud T, Ebbing C, Kessler J, Rasmussen S. Fetal cardiac output, distribution to the placenta and impact of placental compromise. Ultrasound Obstet Gynecol. 2006;28:126‐136. [DOI] [PubMed] [Google Scholar]
- 18. Lewi L, Gratacos E, Ortibus E, et al. Pregnancy and infant outcome of 80 consecutive cord coagulations in complicated monochorionic multiple pregnancies. Am J Obstet Gynecol. 2006;194:782‐789. [DOI] [PubMed] [Google Scholar]
- 19. Van Klink J, Koopman HM, Middeldorp JM, et al. Long‐term neurodevelopmental outcome after selective feticide in monochorionic pregnancies. Br J Obstet Gynaecol. 2015;122:1517‐1524. [DOI] [PubMed] [Google Scholar]
- 20. Chaveeva P, Poon LC, Sotiriadis A, Kosinski P, Nicolaides KH. Optimal method and timing of intrauterine intervention in twin reversed arterial perfusion sequence: case study and meta‐analysis. Fetal Diagn Ther. 2014;35:267‐279. [DOI] [PubMed] [Google Scholar]
- 21. Gaerty K, Greer RM, Kumar S. Systematic review and metaanalysis of perinatal outcomes after radiofrequency ablation and bipolar cord occlusion in monochorionic pregnancies. Am J Obstet Gynecol. 2015;213:637‐643. [DOI] [PubMed] [Google Scholar]
- 22. Van den Bos EM, van Klink JM, Middeldorp JM, Klumper FJ, Oepkes D, Lopriore E. Perinatal outcome after selective feticide in monochorionic twin pregnancies. Ultrasound Obstet Gynecol. 2013;41:653‐658. [DOI] [PubMed] [Google Scholar]
- 23. Kumar S, Paramasivam G, Zhang E, et al. Perinatal‐ and procedure‐related outcomes following radiofrequency ablation in monochorionic pregnancy. Am J Obstet Gynecol. 2014;210:454.e451‐456. [DOI] [PubMed] [Google Scholar]
- 24. Aoki S, Hashimoto K, Ogawa K, Horikawa R, Sago H. Developmental outcomes of Japanese children born through assisted reproductive technology (ART) in toddlerhood. J Obstet Gynaecol Res. 2018;44:929‐935. [DOI] [PubMed] [Google Scholar]
- 25. Cheng S, Maeda T, Tomiwa K, et al. Contribution of parenting factors to the developmental attainment of 9‐month‐old infants: results from the Japan Children's Study. J Epidemiol. 2009;19:319‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salomon LJ, Ortqvist L, Aegerter P, et al. Long‐term developmental follow‐up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin‐to‐twin transfusion syndrome. Am J Obstet Gynecol. 2010;203:444.e441‐447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared yet due to the ongoing nature of the research project.


