Figure 1.
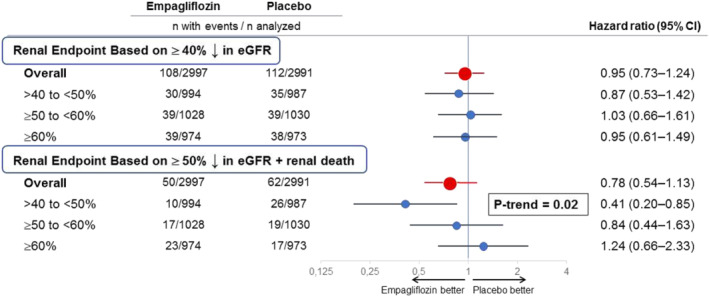
Effect of empagliflozin on major renal outcomes in EMPEROR‐Preserved, overall and in prespecified ejection fraction subgroups, using different definitions for a renal event. Shown are prespecified ejection fraction subgroups: >40% to <50%, ≥50% to <60% and ≥60%. Effect in the overall trial is shown in red, whereas effects in the ejection fraction subgroups are shown in blue. The renal endpoint based on a ≥40% sustained decline in estimated glomerular filtration rate (eGFR) was prespecified in the EMPEROR‐Reduced and EMPEROR‐Preserved trials, whereas the renal endpoint based on a ≥50% sustained decline in eGFR and including renal death was prespecified in the DAPA‐HF trial and was used in a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. 1 Both endpoints included patients who required chronic renal replacement therapy or who experienced a sustained decrease in eGFR to <10–15 mL/min/1.73 m2. There were 14 renal deaths (10 on placebo and 4 on empagliflozin). The P‐trend test refers to the linear influence of three prespecified ejection fraction subgroups on the magnitude of the effect of empagliflozin on major renal outcomes. CI, confidence interval.
