Abstract
Objective
Obesity is associated with impaired gut microbiota diversity, which has been linked to the development of type 2 diabetes. This study aims to examine the effects of an 8‐week aerobic exercise intervention on insulin sensitivity, visceral adiposity, and gut microbiota diversity and composition in participants with obesity.
Methods
Fourteen participants (mean [SD], age 51 [11] years; BMI 34.9 [4.9] kg/m2) performed an 8‐week exercise intervention (2 to 4 times/week on 65% to 85% of heart rate reserve). Insulin sensitivity (hyperinsulemic euglycemic clamp), cardiorespiratory fitness (maximal oxygen uptake), visceral adiposity (dual‐energy X‐ray absorptiometry scan) and gut microbiota composition (16S rRNA gene sequencing) were measured before and after the intervention.
Results
Insulin sensitivity showed a significant increase (pre: 3.8 [1.9] mg/min/kg; post: 4.5 [1.7] mg/min/kg; p = 0.007) after training, whereas visceral adiposity decreased (pre: 959 [361] cm3; post: 897 [364] cm3; p = 0.02). No change in gut microbiota α‐ or β‐diversity was found. At the genus level, the abundance of Ruminococcus gauvreauii (p = 0.02); Lachnospiraceae FCS020 group (p = 0.04), and Anaerostipes (p = 0.04) significantly increased after exercise training. Significant positive correlations were present for M‐value (R. gauvreauii) and VO2 max (R. gauvreauii and Anaerostipes).
Conclusions
Eight‐week exercise training in humans with obesity leads to marked improvements in insulin sensitivity and body composition and is accompanied by modest changes in 3 gut microbiome genera, all belonging to the Firmicutes phylum.
Study Importance.
What is already known?
-
►
Obesity is associated with impaired gut microbiota diversity and composition, which are related to an increased risk of developing type 2 diabetes mellitus and cardiovascular disease.
-
►
Exercise training has been shown to improve gut microbiota diversity.
What does this study add?
-
►
This is the first study that examines exercise‐induced changes in gut microbiota diversity in relation to gold standard measurements of insulin sensitivity.
-
►
This study demonstrates that marked improvements in insulin sensitivity after exercise training are accompanied by modest changes in 3 gut microbiome genera.
How might these results change the direction of research?
-
►
Future research should be directed at elucidating the mechanisms (i.e., circulating factors, short‐chain fatty acids) that cause a rise in insulin sensitivity in response to an exercise stimulus.
INTRODUCTION
Over the past decade, the gut microbiota has emerged as an important modulator of the immune system and energy homeostasis (1). An imbalance (i.e., dysbiosis) in gut microbiota composition in humans has been associated with various metabolic diseases, such as type 2 diabetes mellitus (2, 3). Obesity, a major risk factor for type 2 diabetes mellitus and cardiovascular disease, is associated with gut microbiota dysbiosis that is characterized by decreased diversity and altered composition (4). This highlights the clinical relevance of targeting and improving the gut microbiota in obesity.
In the absence of widely accepted pharmacological therapeutic strategies to improve metabolic health by altering gut microbiota, exercise training may represent a potent therapy. This has been supported by the strong and independent health effects of (regular) exercise training in reducing the risk of type 2 diabetes mellitus by improving insulin sensitivity, especially in untrained individuals with obesity (5, 6, 7). Based on the previously identified link between the gut microbiota and insulin sensitivity, benefits of exercise training on insulin sensitivity may be accompanied by alterations in gut microbiota composition. To support this notion, data from rodent studies have revealed that exercise training in obese mice/rat models improves gut microbiota diversity and composition (8, 9). Voluntary exercise caused an increase in gut microbiota diversity, and these improvements in gut microbiota were linked to enhanced glucose homeostasis (using an oral glucose tolerance test) in mice (8).
In humans, cross‐sectional data suggest that athletes display larger gut microbiota diversity than inactive controls (10), which might be linked to differences in fitness level (11). However, to date, prospective data on the direct effects of exercise training on gut microbiota and metabolic health (i.e., insulin sensitivity) in humans with obesity have been scarce and have displayed heterogenous results (12, 13, 14). Therefore, we examined the impact of an 8‐week aerobic exercise intervention on insulin sensitivity and gut microbiota diversity and composition in individuals with obesity. We hypothesized that exercise training would improve insulin sensitivity and that these changes would be correlated with alterations in gut microbiota.
METHODS
Participants
Twenty inactive participants with obesity (BMI >30 kg/m2) were included in this study. Physical activity levels were assessed with the use of the Short QUestionnaire to ASsess Health‐enhancing physical activity (SQUASH), a validated tool to assess physical activity levels in the Dutch population. Participants were eligible for inclusion when their SQUASH score was 6,400 or lower (15). Participants with inflammatory bowel disease and participants who used pro‐ or antibiotics were excluded from participation because these conditions are associated with an altered gut microbiota (16, 17). Participants with a medical history of diabetes mellitus were excluded from participation. Written informed consent was obtained from all participants prior to the start of the study. This study was approved by the Medical Ethical Committee of the Radboud University Medical Center and was conducted in accordance with the Declaration of Helsinki. This study was registered as NTR5737 in the Netherlands Trial Register. Data represented in this manuscript are a result of a secondary analysis of the primary aim for which the trial was registered. Ethical approval for this specific analysis was obtained.
Study design
All participants who participated in this study were engaged in an 8‐week supervised aerobic exercise training intervention. Participants were instructed not to change dietary habits during the participation in this study. Before and after the intervention, a fresh stool sample was collected by the participants, and a hyperinsulinemic euglycemic clamp, a maximal cycling test, and a dual‐energy X‐ray absorptiometry scan to examine insulin sensitivity, physical fitness levels, and body composition, respectively, were performed.
During this training study, all participants trained 2 to 4 times a week under the supervision of an experienced researcher. Training consisted of cycling exercise on an ergometer (Lode), starting with a 5‐minute warm‐up, followed by 50 minutes of exercise at 65% to 85% of the individual heart rate reserve (HRR), and ending with a cooldown of 5 minutes. Training frequency and percentage of HRR were gradually increased during the first 2 weeks of the intervention period. The HRR was calculated based on individual maximal heart rate assessed during the maximal cycling test. Exercise intensity was continuously monitored and documented with the use of heart rate monitors (Polar), and workload was adjusted accordingly on an individual basis. Participants had to attend at least 95% of the training sessions during the 8‐week intervention period to be eligible for inclusion in the statistical analysis.
Measurements
Anthropometry
At baseline, height and body weight (Seca 888 scale) were measured to calculate BMI. Waist and abdominal circumference was measured with a measuring tape (Seca 201) to calculate waist to hip ratio. Resting heart rate and blood pressure were measured in a supine position, after a 5‐minute rest period. Before and after the training period, a total body dual‐energy X‐ray absorptiometry scan was performed to determine lean body mass and total fat (QDR 4500 densitometer, Hologic Inc.). Visceral adipose tissue (VAT) mass, VAT volume, and VAT area were calculated with standardized Hologic Software with results that correlate excellent with gold standard techniques for the measurement of VAT (18).
Gut microbiota
Participants were provided with a plastic device to collect stool samples, which were stored at −80℃ until DNA extraction. Each participant was instructed to collect a stool sample 48 to 72 hours after cessation of the exercise bout, preferably on a weekday between 6:00 and 11:30 AM in order to quickly store the sample in −80°C at the research facility. Participants collected their stool sample at home and they were asked to hand in the sample as soon as possible. When a participant was not able to travel to the research facility immediately, the stool sample was stored in a fridge at 7°C at the participant’s home. Because most of the participants lived near the research facility and collection time was during working hours, all samples were stored at −80°C within 4 hours after collection. Microbial DNA was isolated from feces using the Maxwell 16 Total RNA system (Promega). Fecal samples were homogenized with 2 beat beating times followed by incubation at 95°C at 100 rpm. Each time, samples were centrifuged for 5 minutes at 4°C and 14,000g to collect the supernatant, which was placed in a new sterile Eppendorf tube. Following this, 250 μL from the obtained supernatant was loaded to the Maxwell 16 Tissue LEV Total RNA Purification Kit (Promega) instrument for DNA extraction. DNA was eluted in 50 μL of nuclease‐free water, and its concentration was quantified using Nanodrop (ThermoScientific). For the amplification of the bacterial 16S ribosomal RNA (rRNA) gene fragment, primers targeting the V5‐V6 region were selected (F784‐R1061). Polymerase chain reaction (PCR) for each sample was performed in triplicate in a total reaction volume of 35 μL. The master mix contained 0.7 μL of the bar‐coded primer (10 µM each per reaction), 0.7 μL of a deoxynucleoside triphosphates (dNTPs) mixture, 0.35 μL of Phusion Green Hot Start II High‐Fidelity DNA Polymerase (2 U/μL; ThermoScientific), 7 μL of 5× Phusion Green HF Buffer, and 25.55 μL of DNAse‐ RNAse‐free water. The amplification program included 30 seconds of initial denaturation step at 98°C, followed by 25 cycles of denaturation at 98°C for 10 seconds, annealing at 42°C for 10 seconds, elongation at 72°C for 10 seconds, and a final extension step at 72°C for 7 minutes. The PCR product was visualized in 1% agarose gel (~290 base pairs [bp]) and purified with the CleanPCR kit (CleanNA). The concentration of the purified PCR products was measured with the Qubit dsDNA BR Assay Kit (Invitrogen), and 200 ng of microbial DNA from each sample was pooled for the generation of the sequencing library. Data filtering and taxonomy assignment were performed using the NG‐Tax pipeline using the default (19). Two distinct in‐house assembled mock communities were included in the library and were compared with their theoretical composition for quality control.
Insulin sensitivity
Peripheral tissue sensitivity to exogenous insulin was measured using a hyperinsulinemic euglycemic clamp as previously described (20). After an overnight fast (10 hours), the participant was placed in the supine position in a quiet, temperature‐controlled (22°C‐24°C) room. Insulin (Novorapid, Novo‐Nordisk) was infused intravenously in a dose of 430 pmol·m−2·min−1 (60 mU · m−2 · min−1) for 120 minutes. Insulin (50 U/mL) was diluted in 47.5 mL of NaCl 0.9% with the addition of 2 mL of blood from the participant to a concentration of 1 U/mL. Venous plasma glucose concentrations were clamped at 5.0 mmol/L by a variable glucose 20% infusion rate, adjusted depending on venous plasma glucose levels measured at 5‐minute intervals. Serum glucose levels were determined using a Biosen C‐Line Glucose and Lactate Analyser (Biosen C‐line GP+, EKF‐diagnostic GmbH). Whole‐body glucose disposal was calculated as the mean glucose infusion rate per kilogram body weight (mg/kg/min) during the last 30 minutes of the clamp (M‐value). The hyperinsulemic euglycemic clamp after the training period was performed at least 72 hours after the last exercise bout.
Dietary intake
During the training intervention, participants were instructed not to change their dietary habits. To assess potential changes in daily food intake, participants were asked to record dietary intake before and in the last week of the training intervention in a detailed food journal. Participants were individually instructed on how to record food items and were provided with sample diaries. Dietary records of the 24 hours prior to stool collection were analyzed with Eetmeter Software (Voedingscentrum), based on the Dutch Food Composition Database of 2016 (21). Furthermore, an online, validated, 180‐item, semiquantitative Food Frequency Questionnaire was used to assess habitual daily energy intake and macronutrient intake (22, 23). The Food Frequency Questionnaire reference period was 1 month, and portion sizes were estimated using standard portions (24). Intake of total energy and nutrients was calculated using the Dutch Food Composition Database (21).
Cardiorespiratory fitness level
Participants performed a maximal exercise test on an electrically braked leg‐cycling ergometer (Lode Excalibur) using an incremental protocol to assess their cardiorespiratory fitness level. Workload increased by 10 to 30 W per minute, starting at 0 W, until exhaustion. A calibrated gas analyzer was used to measure oxygen consumption continuously (COSMED Pulmonary Function Equipment). During the test, an electrocardiogram (ECG) was continuously recorded and checked by a physician. The maximal exercise test was terminated by adhering to the guidelines of the American Heart Association (25). Maximal oxygen consumption (VO2 max) was defined as the highest oxygen uptake (30‐second average).
Statistical analysis
All statistical analyses were conducted in SPSS Statistics version 22 (IBM Corp.). Data were checked for normality with use of the Shapiro–Wilk test. Participant characteristics were normally distributed and therefore assessed with use of a paired t test to examine the impact of exercise training. Correlations between measures of alpha diversity and abundance of gut microbiota versus participant characteristics were assessed with use of repeated measures correlation (Rmcorr) (26). The level of statistical significance was defined at α = 0.05. Data are presented as mean (SD), unless stated otherwise.
Microbial data analysis
Alpha and beta diversity analyses were performed and visualized using the publicly available Microbiome R package (version 1.2.1) (27). Alpha diversity analyses provide information about richness (number of species) and/or evenness (relative abundance of those species) within a sample (28). Alpha diversity was determined by Chao index (nonparametric estimation of species richness) (29), Shannon index (measuring richness and evenness by taking relative abundance into account) (30), and Faith’s index (PD, phylogenetic diversity: the sum of the branch lengths of the phylogenetic tree, a measurement of diversity in taxon subsets) (31). Beta diversity analyses provide information about variation between samples (28). Beta diversity was calculated using the Bray–Curtis dissimilarity index and visualized through a principal coordinates analysis. The Envfit function from the Vegan package that fits environmental vectors or factors onto an ordination was used to evaluate whether age, sex, body mass (kilograms), insulin sensitivity (M‐value), BMI, cardiorespiratory fitness (VO2 max), VAT volume, and dietary measures (daily intake of kilocalories, fat, saturated fat, carbohydrate, protein) were associated with the nonmetric dimensional scaling (NMDS) ordinations; i.e., could explain the variance observed in the data set. The significance of the fitted factors was estimated using 999 permutations. Repeated measures correlations, designed for paired samples, were performed using the Rmcorr package, to assess correlations between environmental variables and bacterial taxa (26). The Wilcoxon signed rank test was used to examine whether significant changes in gut microbiota occurred on the genus/family/order/class level.
RESULTS
Effect of training intervention
Participant characteristics before and after training are presented in Table 1. Twenty participants (11 women, 9 men) completed the exercise intervention. Because 2 participants were unable to collect a stool sample before the start of the intervention and 4 additional participants were unable to collect a stool sample in the given time frame after the intervention, these were excluded from analysis, leading to a sample size of n = 14 participants (7 women, 7 men) with samples collected before and after training. Data were analyzed for this subgroup. Training compliance for this subgroup was 98% (Figure 1). Characteristics from this subgroup (n = 14) were not different from the entire cohort (data not shown).
TABLE 1.
Physiological characteristics before and after the exercise intervention of the subgroup (n = 14) with available microbiota data at both time points
| Subgroup analysis (n = 14) | p value | ||
|---|---|---|---|
| Pre | Post | ||
| Age (y) | 51 ± 11 | ‐ | ‐ |
| Female sex (%) | 50% | ||
| Body composition | |||
| Weight (kg) | 105.4 ± 16.8 | 102.6 ± 17.4 | 0.03 |
| BMI (kg/m²) | 34.9 ± 4.9 | 33.9 ± 5.2 | 0.03 |
| Waist to hip ratio | 1.00 ± 0.10 | 0.99 ± 0.07 | 0.36 |
| VAT mass (g) | 887 ± 334 | 830 ± 337 | 0.04 |
| VAT volume (cm3) | 959 ± 361 | 897 ± 364 | 0.02 |
| Insulin sensitivity | |||
| M‐value (mg/min/kg) | 3.8 ± 1.9 | 4.5 ± 1.7 | 0.007 |
| Blood pressure | |||
| Systolic blood pressure (mmHg) | 132 ± 16 | 131 ± 14 | 0.6 |
| Diastolic blood pressure (mmHg) | 87 ± 11 | 80 ± 9 | 0.003 |
| Resting heart rate (bpm) | 67 ± 10 | 72 ± 12 | 0.5 |
| Physical fitness | |||
| VO2 max (mL/min/kg) | 27.7 ± 5.5 | 31.9 ± 7.0 | <0.0001 |
| VO2 max (mL/min/kg FFM) | 46.0 ± 6.6 | 51.2 ± 8.0 | 0.001 |
| Power (W) | 204 ± 42 | 250 ± 47 | <0.001 |
| Daily dietary composition | |||
| Energy intake (kcal) | 2,028 ± 622 | 1,905 ± 389 | 0.18 |
| Carbohydrate (g) | 211 ± 39 | 210 ± 58 | 0.94 |
| Fat (g) | 75 ± 42 | 66 ± 22 | 0.28 |
| Unsaturated fat (g) | 28 ± 18 | 26 ± 9 | 0.74 |
| Protein (g) | 98 ± 26 | 93 ± 18 | 0.52 |
| Lipid profile | |||
| Cholesterol (mmol/L) | 5.6 ± 1.5 | 5.2 ± 1.1 | 0.03 |
| HDL (mmol/L) | 1.4 ± 0.3 | 1.4 ± 0.3 | 0.35 |
| LDL (mmol/L) | 3.7 ± 1.3 | 3.4 ± 1.2 | 0.05 |
| Triglycerides (mmol/L) | 1.8 ± 0.7 | 1.8 ± 0.8 | 0.99 |
Data given as mean ± SD. p value represents the level of significance for post versus pre values.
Abbreviations: FFM, fat free mass; HDL, high density lipoprotein; LDL, low density lipoprotein; VAT, visceral adipose tissue.
FIGURE 1.
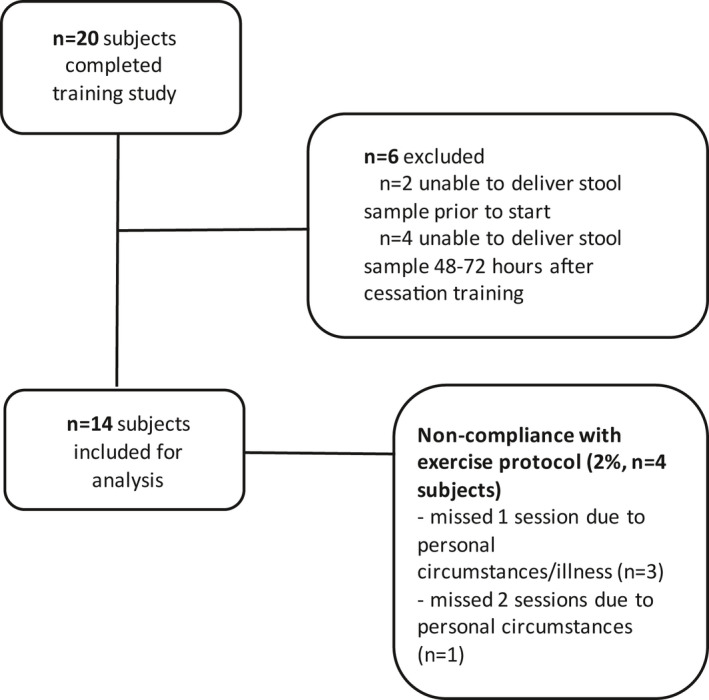
CONSORT (Consolidated Standards of Reporting Trials) diagram of excluded participants and exercise training compliance
Assessment of dietary intake by food journals showed no significant change in daily energy intake (pre: 2,028 ± 622 kcal/d; post: 1,906 ± 390 kcal/d; p = 0.18) or in macronutrient composition before and at the end of the intervention period (Table 1). Cardiorespiratory fitness levels, insulin sensitivity (M‐value), and body composition improved significantly (Table 1).
Gut microbiota
Alpha diversity
No change in alpha (α)‐diversity of the gut microbiota was observed after training, as assessed by Shannon index, phylogenetic diversity index, and Chao index (Figure 2A).
FIGURE 2.

Alpha and beta diversity before and after the exercise intervention. (A) Alpha diversity before and after the exercise intervention: i. Shannon index; ii. PD; iii. Chao1. (B) Beta diversity (Bray–Curtis) before ( ) and after (
) and after ( ) the exercise intervention. NMDS, non‐metric dimensional scaling; PD, phylogenetic diversity [Colour figure can be viewed at wileyonlinelibrary.com]
) the exercise intervention. NMDS, non‐metric dimensional scaling; PD, phylogenetic diversity [Colour figure can be viewed at wileyonlinelibrary.com]
Beta diversity
Bray–Curtis analysis showed that samples did not cluster by time (pre‐ versus post‐training) and that, consistently, beta (β)‐diversity did not change in post‐ versus pre‐training (Figure 2B).
Composition
On the genus level, a total number of 3 taxa showed a significant change after the exercise intervention: Ruminococcus gauvreauii (p = 0.02), uncultured genus from Lachnospiraceae (p = 0.04), and Anaerostipes (p = 0.04) (Figure 3). On the family, phylum, class, and order levels, no significant change in taxa was found after the 8‐week exercise training intervention.
FIGURE 3.
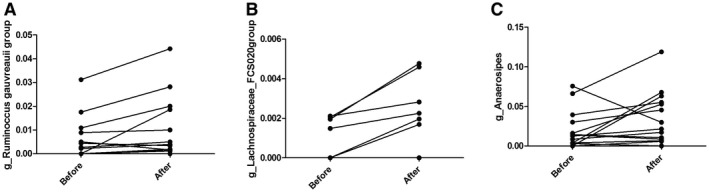
Significant changes in genus (A) Ruminococcus gauvreauii, (B) Lachnospiraceae FCS020, and (C) Anaerostipes before and after the exercise intervention. Y‐axis represents relative abundance of each genus
Envfit analysis showed that only body mass showed borderline significance explaining the total variation in gut microbiota composition (p = 0.05), whereas the improvement in VO2 max (i.e., effect size of the exercise training intervention) and the other potential explanatory variables (i.e., sex, age, M‐value, BMI, VO2 max, VAT volume, and dietary intake measures) did not explain significantly the differences in microbial composition of the participants before and after the intervention.
Correlation analysis
For this analysis, both samples collected prior to and after the exercise intervention were used. To examine the relationship between gut microbiota composition and participant characteristics (insulin sensitivity, cardiorespiratory fitness, body composition measures, and dietary intake measures) further, the top 30 most abundant bacterial genera were correlated with M‐value, VO2 max, body mass, BMI, VAT volume, caloric intake, and intake of fat, carbohydrate, and protein. The abundance of Anaerostipes was strongly positively correlated with VO2 max (r = 0.64, p = 0.015). The abundance of Ruminococcus2 was positively correlated with VAT volume (r = 0.51, p = 0.0048). The abundance of R. gauvreauii group was positively correlated with M‐value (r = 0.60, p = 0.023) and VO2 max (r 2 = 0.61, p = 0.0018) and negatively with VAT volume (r = −0.54, p = 0.028) (Figure 4). The abundance of Subdoligranulum was negatively correlated with intake of fat (r = −0.59, p = 0.035) and caloric intake (r = −0.62, p = 0.021). The abundance of both Roseburia and Eubacterium hallii group was negatively correlated with carbohydrate intake (r = −0.61, p = 0.026 and r = −0.58, p = 0.04, respectively).
FIGURE 4.
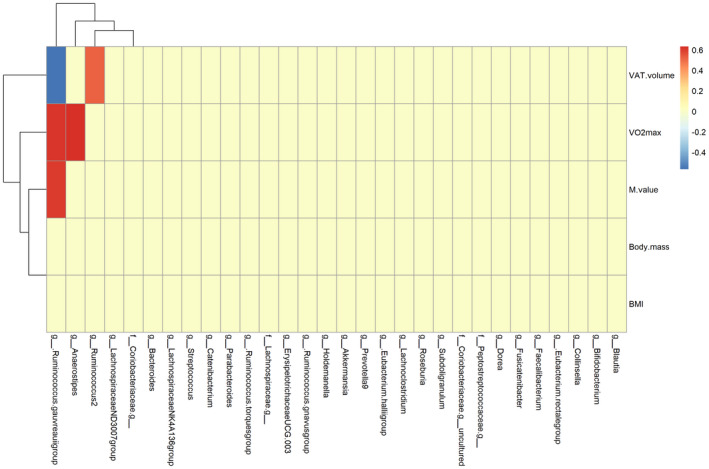
Correlations between participant characteristics (M‐value, body mass, BMI, VAT volume, and VO2 max) and the 30 most abundant genera of gut microbiota in fecal samples (data from before and after the exercise intervention). VAT, visceral adipose tissue [Colour figure can be viewed at wileyonlinelibrary.com]
No significant correlations between the different measures of α‐diversity (i.e., Shannon index, Phylogenetic diversity index, and Chao 1) and changes in body composition (body weight, BMI, VAT mass), insulin sensitivity (M‐value), or cardiorespiratory fitness (VO2 max) were found (data not shown).
DISCUSSION
This study presents the following findings. First, an 8‐week aerobic exercise intervention in humans with obesity led to marked improvements in insulin sensitivity and body composition, whereas this was not accompanied by improvements in gut microbiota α‐ and β‐diversity. Modest but significant changes in 3 genera (R. gauvreauii, uncultured Lachnospiraceae, and Anaerostipes) after the exercise intervention were found. Of these genera, R. gauvreauii and Anaerostipes both showed a significantly positive correlation with VO2 max. R. gauvreauii also correlated positively with M‐value and negatively with VAT volume. This suggests that gut microbiota exerts adaptability in response to exercise training, which might be associated with improvements in metabolic and cardiovascular health.
To demonstrate the impact of exercise training, precise, high‐quality techniques were used for the measurement of insulin sensitivity (20), VAT (18), and cardiorespiratory fitness (25). In line with our hypothesis, and reinforced by several previous studies (5), large beneficial effects of exercise training on M‐value, VAT, and fitness levels were observed. This proves that the 8‐week exercise intervention performed in this study is a successful tool in improving risk factors for the development of metabolic and cardiovascular disease. After 8 weeks of effective exercise training, no change in gut microbiota diversity was found in our cohort of individuals with obesity. The lack of exercise‐induced alterations in α‐ and β‐diversity is in accordance with previous human exercise intervention studies of both shorter (3 weeks) and longer (12 weeks) duration and similar exercise intensities (12, 14). This is in contrast to cross‐sectional work in athletes that demonstrated marked differences in the gut microbiota diversity when compared with inactive controls, suggesting a role for exercise as an influencer of gut microbiota diversity (32). Indeed, 2 exercise studies found alterations in β‐diversity of the gut microbiota (33, 34). In a study by Allen et al., these alterations were dependent on obesity status: In lean participants, exercise‐induced shifts in bacterial taxa were more pronounced than in individuals with obesity (33). This suggests that gut microbiota diversity in humans with obesity might be more rigid and unable to respond to an exercise stimulus. As in our study, participants in Allen et al. were instructed to maintain their regular dietary intake to discard the influence of a change in diet on gut microbiota. Moreover, the exercise intervention was of similar intensity (60% to 75% HRR) and duration (6 weeks) (33). Therefore, the differences between our study and others are unlikely the result of a different exercise design. More likely, other factors might play a role, such as lifelong training status and childhood dietary regimen, which could also explain the large differences observed in cross‐sectional comparison of elite athletes to sedentary controls. At least, this suggests that exercise‐mediated improvements in insulin sensitivity occur independently of changes in gut microbiota diversity.
In this study, modest but significant changes in gut microbiota composition occurred. On the genus level, abundance of R. gauvreauii group, Lachnospiraceae FCS020 group, and Anaerostipes was increased after exercise training. Interestingly, R. gauvreauii was also positively correlated with insulin sensitivity (M‐value) and cardiorespiratory fitness levels (VO2 max) and inversely correlated with visceral adiposity. R. gauvreauii is derived from the order of Clostridium in the phylum of Firmicutes (35). Its abundance was decreased in patients with coronary artery disease (CAD) when compared with controls (36). VO2 max is a strong, independent risk factor for the development of cardiovascular disease. The exercise‐induced increase in R. gauvreauii and its positive correlation with VO2 max we found in our study, together with the observation that its abundance is lower in CAD patients, suggest that exercise might be capable of improving cardiovascular risk mediated by altering gut microbiota in individuals with obesity. R. gauvreauii produces acetate as an end product of fermentation (35). Acetate is a short‐chain fatty acid (SCFA) that elicits various beneficial effects on other tissues in the body, ultimately improving body weight control and insulin sensitivity (37). This is in accordance with our results that demonstrate a positive correlation between R. gauvreauii and gold standard measurements of insulin sensitivity and a negative correlation with VAT mass. Taken together, our results suggest that exercise‐induced improvements in glucose homeostasis might be associated with an increase in acetate‐producing R. gauvreauii.
This study also demonstrates a modest increase in Anaerostipes derived from the family of Lachnospiraeceae in the phylum Firmicutes, in the presence of a positive correlation with VO2 max. Anaerostipes is a butyrate producer by lactate utilization (38). Its abundance has not been described to be altered by exercise interventions in humans with obesity in previous studies. However, Rettedal et al. found that its abundance was higher in lean participants compared with participants with obesity (37). The correlation with VO2 max we found in our study suggests its adaptability to an exercise stimulus toward a more favorable “lean” phenotype. However, it can also be a direct consequence of the lactate shifts that result from multiple strenuous exercise interventions. Lastly, we also found an increase in the genus Lachnospiraceae FCS020 group, also derived from the family of Lachnospiraeceae in the phylum Firmicutes. Data on Lachnospiraceae FCS020 group in humans are scarce. It has been associated with circulating very low‐density lipoprotein and small high‐density lipoprotein particles and plasma trimethylamine N‐oxide, all potential risk factors for CAD (39, 40). In our study, its abundance was not correlated to any of the established cardiovascular risk factors (i.e., insulin resistance or visceral adiposity). Therefore, it remains unknown what the clinical significance of this change is. Taken together, our study demonstrates that exercise training increases the abundance of 2 SCFA‐producing genera belonging to the Firmicutes phylum that are associated with improvements in cardiorespiratory fitness levels and insulin sensitivity. This suggests that exercise‐induced improvements in cardiometabolic health might be mediated by SCFA‐producing gut microbiota. Future work is required to directly study this hypothesis.
Some methodological considerations must be taken into account in our study. First, although based on previous work showing that SCFAs produced by bacterial taxa from the Firmicutes phylum might play a role in exercise‐induced improvements in insulin sensitivity, we were unable to measure these in the stool samples of our participants. Unfortunately, this was not part of the original research design. This should be incorporated in future studies examining this topic. Second, the gut microbiota data in our cohort showed a large interindividual variance, which is in accordance with large cohort microbiota studies in humans (41, 42) and also smaller human intervention studies (12). Nonetheless, our primary comparison involves intraindividual changes, which adds strength to our observation that exercise training did not alter the gut microbiota. Third, the timing of gut microbiota measurement (i.e., collection of the stool sample) is an important factor potentially affecting results as a temporarily dysbiosis in gut microbiota after strenuous exercise can occur (43). Because all participants collected a stool 48 to 72 hours after cessation of the last exercise bout to rule out acute effects of the last exercise bout, this minimized the potential impact of the last exercise bout on gut microbiota measures. Lastly, a change in diet is known to cause an alteration in gut microbiota (44). Therefore, participants were carefully instructed not to change caloric and macronutrient intake, which was objectively reported with the use of food diaries. Because our data demonstrated that diet had not changed, we can exclude changes in diet as a potential factor influencing our results. This is further supported by the Envfit analysis, which showed that macronutrient and caloric intake did not influence the variation in change of gut microbiota composition, and the correlation analysis, in which no significant correlation was found between the 3 significantly altered genera after exercise training and dietary intake measures.
This study demonstrated that an 8‐week exercise intervention in humans with obesity causes significant improvements in cardiovascular and metabolic health in the presence of modest changes in 3 gut microbiome genera, all belonging to the SCFA‐producing Firmicutes phylum. Of these genera, R. gauvreauii is positively correlated with insulin sensitivity and cardiorespiratory fitness, which suggests a potential role for this acetate producer to cause improvement in insulin sensitivity in response to exercise.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
CLINICAL TRIAL REGISTRATION
Netherlands Trial Register NTR5737 (www.trialregister.nl).
Verheggen RJHM, Konstanti P, Smidt H, Hermus ARMM, Thijssen DHJ, Hopman MTE. Eight‐week exercise training in humans with obesity: Marked improvements in insulin sensitivity and modest changes in gut microbiome. Obesity (Silver Spring). 2021;29:1615–1624. 10.1002/oby.23252
DATA AVAILABILITY STATEMENT
Data of individual participants (after deidentification), study protocol, informed consent forms, and statistical analysis plan will be available from 3 months to 36 months after publication of the article to researchers who provide a methodologically sound proposal for the purpose of a meta‐analysis with individual data. Requests and proposals can be directed to rebecca.verheggen@radboudumc.nl.
REFERENCES
- 1. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027‐1031. [DOI] [PubMed] [Google Scholar]
- 2. Qin J, Li Y, Cai Z, et al. A metagenome‐wide association study of gut microbiota in type 2 diabetes. Nature. 2012;4:55‐60. [DOI] [PubMed] [Google Scholar]
- 3. Kostic A, Gevers D, Siljander H, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. JAMA. 2001;286:1218‐1227. [DOI] [PubMed] [Google Scholar]
- 6. Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343‐1350. [DOI] [PubMed] [Google Scholar]
- 7. Shaw KA, Gennat HC, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD003817. doi: 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans CC, LePard KJ, Kwak JW, et al. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet‐induced obesity. PLoS One. 2014;9:e92193. doi: 10.1371/journal.pone.0092193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Petriz BA, Castro AP, Almeida JA, et al. Exercise induction of gut microbiota modifications in obese, non‐obese and hypertensive rats. BMC Genom. 2014;15:511. doi: 10.1186/1471-2164-15-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clarke SF, Murphy EF, O'Sullivan O, et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63:1913‐1920. [DOI] [PubMed] [Google Scholar]
- 11. Estaki M, Pither J, Baumeister P, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. 2016;4:42. doi: 10.1186/s40168-016-0189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu Y, Wang Y, Ni Y, et al. Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab. 2020;31:77‐91.e75. [DOI] [PubMed] [Google Scholar]
- 13. Munukka E, Ahtiainen JP, Puigbó P, et al. Six‐week endurance exercise alters gut metagenome that is not reflected in systemic metabolism in over‐weight women. Front Microbiol. 2018;9:2323. doi: 10.3389/fmicb.2018.02323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rettedal EA, Cree JME, Adams SE, et al. Short‐term high intensity interval training (HIIT) exercise does not affect gut bacterial community diversity or composition of lean and overweight men. Exp Physiol. 2020;105:1268‐1279. [DOI] [PubMed] [Google Scholar]
- 15. Wendel‐Vos GC, Schuit AJ, Saris WH, Kromhout D. Reproducibility and relative validity of the short questionnaire to assess health‐enhancing physical activity. J Clin Epidemiol. 2003;56:1163‐1169. [DOI] [PubMed] [Google Scholar]
- 16. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1‐10. [DOI] [PubMed] [Google Scholar]
- 17. Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013;13:889‐899. [DOI] [PubMed] [Google Scholar]
- 18. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual‐energy X‐ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring). 2012;20:1109‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramiro‐Garcia J, Hermes G, Giatsis C, et al. NG‐Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes. F1000 Research. 2016;5:1791. doi: 10.12688/f1000research.9227.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214‐E223. [DOI] [PubMed] [Google Scholar]
- 21. RIVM/Voedingscentrum NEVO‐tabel 2016. http://nevo‐online.rivm.nl/. 2017. Accessed December 16, 2020.
- 22. Streppel MT, de Vries JHM, Meijboom S, et al. Relative validity of the food frequency questionnaire used to assess dietary intake in the Leiden Longevity Study. Nutr J. 2013;12:75. doi: 10.1186/1475-2891-12-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siebelink E, Geelen A, de Vries JH. Self‐reported energy intake by FFQ compared with actual energy intake to maintain body weight in 516 adults. Br J Nutr. 2011;106:274‐281. [DOI] [PubMed] [Google Scholar]
- 24. van der Heijden L. Maten, gewichten en codenummer 2003. In: Informatorium VOeding en Diëtitiek ‐ Voedingsleer. Former M, ed. Houten: Bohn Stafleu van Loghum; 2013. [Google Scholar]
- 25. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873‐934. [DOI] [PubMed] [Google Scholar]
- 26. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. doi: 10.3389/fpsyg.2017.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shetty SA, Lahti L. Microbiome data science. J Biosci. 2019;44:115. doi: 10.1007/s12038-019-9930-2 [DOI] [PubMed] [Google Scholar]
- 28. Kim B‐R, Shin J, Guevarra RB, et al. Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol. 2017;27:2089‐2093. [DOI] [PubMed] [Google Scholar]
- 29. Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11:265‐270. [Google Scholar]
- 30. Lemos LN, Fulthorpe RR, Triplett EW, Roesch LF. Rethinking microbial diversity analysis in the high throughput sequencing era. J Microbiol Methods. 2011;86:42‐51. [DOI] [PubMed] [Google Scholar]
- 31. Faith DP. Conservation evaluation and phylogenetic diversity. Biol Conserv. 1992;61:1‐10. [Google Scholar]
- 32. Barton W, Penney NC, Cronin O, et al. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67:625‐633. [DOI] [PubMed] [Google Scholar]
- 33. Allen JM, Mailing LJ, Niemiro GM, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50:747‐757. [DOI] [PubMed] [Google Scholar]
- 34. Cronin O, Barton W, Skuse P, et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems. 2018;3:e00044‐18. doi: 10.1128/mSystems.00044-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Domingo MC, Huletsky A, Boissinot M, Bernard KA, Picard FJ, Bergeron MG. Ruminococcus gauvreauii sp. nov., a glycopeptide‐resistant species isolated from a human faecal specimen. Int J Syst Evol Microbiol. 2008;58:1393‐1397. [DOI] [PubMed] [Google Scholar]
- 36. Toya T, Corban MT, Marrietta E, et al. Coronary artery disease is associated with an altered gut microbiome composition. PLoS One. 2020;15:e0227147. doi: 10.1371/journal.pone.0227147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hernández MAG, Canfora EE, Jocken JWE, Blaak EE. The short‐chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11:1943. doi: 10.3390/nu11081943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwiertz A, Hold GL, Duncan SH, et al. Anaerostipes caccae gen. nov., sp. nov., a new saccharolytic, acetate‐utilising, butyrate‐producing bacterium from human faeces. Syst Appl Microbiol. 2002;25:46‐51. [DOI] [PubMed] [Google Scholar]
- 39. Diling C, Longkai QI, Yinrui G, et al. CircNF1‐419 improves the gut microbiome structure and function in AD‐like mice. Aging (Albany NY). 2020;12:260‐287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40. Gao J, Yan K‐T, Wang J‐X, et al. Gut microbial taxa as potential predictive biomarkers for acute coronary syndrome and post‐STEMI cardiovascular events. Sci Rep. 2020;10:2639. doi: 10.1038/s41598-020-59235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. The Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peters HP, De Vries WR, Vanberge‐Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001;48:435‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data of individual participants (after deidentification), study protocol, informed consent forms, and statistical analysis plan will be available from 3 months to 36 months after publication of the article to researchers who provide a methodologically sound proposal for the purpose of a meta‐analysis with individual data. Requests and proposals can be directed to rebecca.verheggen@radboudumc.nl.


