Abstract
Barth syndrome is an X‐linked disorder characterized by cardiomyopathy, skeletal myopathy, and neutropenia, caused by deleterious variants in TAFAZZIN. This gene encodes a phospholipid‐lysophospholipid transacylase that is required for the remodeling of the mitochondrial phospholipid cardiolipin (CL). Biochemically, individuals with Barth syndrome have a deficiency of mature CL and accumulation of the remodeling intermediate monolysocardiolipin (MLCL). Diagnosis typically relies on mass spectrometric measurement of CL and MLCL in cells or tissues, and we previously described a method in blood spot that uses a specific MLCL/CL ratio as diagnostic biomarker. Here, we describe the evolution of our blood spot assay that is based on the implementation of reversed phase‐UHPLC separation followed by full scan high resolution mass spectrometry. In addition to the MLCL/CL ratio, our improved method also generates a complete CL spectrum allowing the interrogation of the CL fatty acid composition, which considerably enhances the diagnostic reliability. This addition negates the need for a confirmatory test in lymphocytes thereby providing a shorter turn‐around‐time while achieving a more certain test result. As one of the few laboratories that offer this assay, we also evaluated the diagnostic yield and performance from 2006 to 2021 encompassing the use of both the original and improved assay. In this period, we performed 796 diagnostic analyses of which 117 (15%) were characteristic of Barth syndrome. In total, we diagnosed 93 unique individuals with Barth syndrome, including three females, which together amounts to about 40% of all reported individuals with Barth syndrome in the world.
Keywords: Barth syndrome, biomarkers, cardiolipins, dried blood spot testing, inborn errors of metabolism, mass spectrometry
1. INTRODUCTION
Barth syndrome (BTHS, MIM 302060) is an X‐linked disorder that is characterized by cardiomyopathy, skeletal myopathy, and neutropenia. For a detailed overview of the clinical symptoms, see review in this issue 1 and Ferreira et al. 2 The affected gene, TAFAZZIN (previously TAZ, G4.5), encodes a phospholipid‐lysophospholipid transacylase involved in the remodeling of the mitochondrial phospholipid cardiolipin (CL). 3 CL is a mitochondrial lipid that is highly concentrated in the inner mitochondrial membrane, and its unique physicochemical properties facilitate the characteristic curvature of the inner mitochondrial membrane where CL plays a crucial role in the respiratory chain, transport of metabolites across the IMM, and in the initiation of type II apoptosis. CL consists of three glycerol molecules bridged by two phosphates where the outer two glycerol moieties carry a total of four fatty acid side‐chains. CL remodeling is required given that the primary CL synthesis enzyme CL synthase does not achieve the highly specific acyl chain composition that is vital for its function. 4 Therefore, upon synthesis of the nascent/immature CL, tafazzin ensures remodeling toward the primarily linoleic acid‐enriched—that is, polyunsaturated—composition of mature CL.
The biochemical consequences of tafazzin deficiency are that the remodeling precursor monolysocardiolipin (MLCL, with three fatty acid side chains) accumulates, and the remaining CL species are more saturated and contain much less linoleic acid, but instead have mainly monounsaturated fatty acids (mainly oleic and palmitoleic acid). In 2006, we described a mass spectrometric assay in blood spot for the biochemical screening of BTHS based on the ratio between one species of MLCL and one species of CL. 5 By choosing the highest accumulating MLCL in the numerator of the ratio (MLCL(52:2)), and the most profoundly deficient CL as the denominator (CL(72:8)), the ratio is highly elevated in case of BTHS and serves as a powerful and specific (derived) biomarker. This method employed a hyphenated approach; first separating the analytes in the lipid extract by normal‐phase HPLC followed by quantification of the abundance of the two lipid species by specific transitions using a triple‐quadrupole mass spectrometer. This method was successful, but a subsequent confirmatory HPLC‐MS analysis in lymphocytes or fibroblasts was advised to confirm the blood spot screening result. In the lymphocyte extract, in addition to determining the specific MLCL/CL ratio in lymphocytes, the complete (ML)CL spectrum was evaluated where the characteristic shift to more saturated species further confirmed the BTHS diagnosis. 6 , 7
Based on this concept, we developed a revised method to screen for BTHS that uses a robust reversed‐phase system in combination with full scan high resolution Orbitrap mass spectrometry. This new assay can be applied to blood spots, as well as lymphocytes and fibroblasts, and now simultaneously allows the measurement and evaluation of the ML(CL) spectrum making the confirmatory and more logistically complex and expensive analysis in lymphocytes/fibroblasts superfluous. As one of the few laboratories in the world performing this assay we have analyzed samples from ~40% of the 230‐250 cases known worldwide 8 and therefore we also evaluated the diagnostic yield and performance of the assay since its introduction in 2006.
2. METHODS
2.1. Determination of the MLCL/CL ratio by high resolution mass spectrometry
A punch (quarter‐inch diameter) of a dried bloodspot on filter paper (Guthrie card) was transferred to a 2 mL tube, to which was added 1 mL methanol/chloroform (1:1, vol/vol). After the addition of 5 μL of 10 μmoL/L CL(14:0)4 = CL(56:0) (Avanti Polar Lipids, Alabaster, AL), the internal standard, the sample was vortex‐mixed and incubated for 15 minutes at room temperature in a sonicator bath (Branson 3510). The extraction fluid, with the filter paper removed, was transferred to a 4 mL glass tube and evaporated to dryness (60°C, N2). The residue was reconstituted in 50 μL methanol and transferred to a sample vial, and capped. We used two injections of 10 μL for UHPLC‐mass spectrometry (UHPLC‐MS) analysis. Chromatographic separation was achieved on a Dionex UltiMate 3000 Rapid Separation system (Thermo Fisher Scientific, Waltham, MA) equipped with the following column; Acquity HSS T3, 2.1 × 100 mm, 1.8 μm particle size (Waters, Massachusetts, MA). Samples were eluted on a gradient between eluant A (H2O/Methanol = 6/4 + 10 mM ammoniumformate +0.1% formic acid) and eluant B (isopropanol/Methanol = 9/1 + 10 mM ammoniumformate +0.1% formic acid) in 5 minutes (t = 0 flow 0.4 mL/min 50%B, t = 1 70%B, t = 6.5100%B, t = 8.5100%B, t = 8.6 50%B, t = 9.6 next injection). We performed mass spectrometry analyses on a Q Exactive plus (Thermo Fisher Scientific, Waltham, MA) operated in the negative ion electrospray ionization (ESI) mode using MS1 scanning and monitoring the following ions; CL(72:8): m/z 1447.9650, CL(56:0); m/z 1239.8398, MLCL(52:2); m/z 1165.7666. In the first injection, we collected data to calculate the ratio. To obtain a better signal to noise ratio, we used small scan ranges for the extracted chromatograms, scan range m/z 1441‐1465 for CL(72:8), 1239‐1245 for CL(56:0), and 1159‐1170 for MLCL(52:2). We generated extracted ion chromatograms for the three exact masses and integrated the chromatographic peak to obtain the abundance of each metabolite. The ratio between MLCL(52:2) and CL(72:8) was calculated and termed the MLCL/CL ratio. As MLCL frequently was undetectable yielding a ratio of zero, we set ratios below 0.01 to a value of 0.01 to allow graphical representation and statistical analysis. To estimate the CL(72:8) or MLCL(52:2) concentrations, their abundances were divided by that of the internal standard CL(56:0) and this response was multiplied by the internal standard concentration.
In a second injection, we scanned the range m/z 1000‐1600 to measure the complete spectrum of all MLCL and CL species. Although the signal to noise ratio for individual MLCL and CL species is worse compared to the three miniscans obtained in the first injection, it gives a comprehensive overview of the potential shift to CL species with smaller and more saturated fatty acid chains that is characteristic of BTHS. The spectrum was always checked visually to ascertain the likelihood of a BTHS diagnosis, especially if the MLCL/CL ratio was more than 0.2.
2.2. Validation of the MLCL/CL assay
Intra‐assay precision: control and BTHS blood spots were prepared by pipetting 40 μL of whole blood on a Guthrie card. For each level, five spots were punched, extracted and the MLCL/CL ratio was determined as described above.
Inter‐assay precision: two control and one BTHS blood spot were made as for intra‐assay precision. On five different days, for controls and BTHS, a blood spot was punched, extracted, and the MLCL/CL ratio was determined.
Instrument precision: a control and BTHS blood spot extract was prepared and injected five times from the same extract and the MLCL/CL ratio was determined.
Linearity was estimated by using blood spots of an (anonymized) control and one BTHS individual as MLCL(52:2) is not available commercially and we wanted to test linearity in the blood spot matrix. For each of the six concentration levels (each measured n = 4), a total of five blood spots were extracted where highest MLCL level (level 1) contained five BTHS blood spots and the highest CL level (level 6) contained five control blood spots. Levels 2, 3, 4, and 5 contained 4, 3, 2, 1 of the BTHS blood spots together with 1, 2, 3, and 4 control blood spots, respectively.
In accordance with the research code of the Amsterdam UMC, no ethics approval was needed as this was an (anonymized) retrospective study of patient samples that were sent to us for BTHS diagnostics.
Clinical validation: Blood spot samples were collected under Johns Hopkins University IRB protocol “NA_00090474 Multidisciplinary Studies in Barth Syndrome” (H.V. is principal investigator). Blood spots were collected from males ages 24 months to 32 years 7 months, all of whom had molecular confirmation of a pathogenic variant in the TAFAZZIN gene. All blood samples were drawn at 3‐4 hours of fasting to ensure uniform dietary effects on metabolite profiles.
Leukocyte count correlation with CL(72:8): to determine the dependence of the leukocyte count of blood used to make bloodspots on the levels of CL(72:8) we collected anonymized EDTA blood samples of non‐BTHS individuals with known leukocyte counts and made blood spots. Leukocytes counts were chosen to span from the normal range to considerable leukocytosis and a linear correlation analysis was performed with the concentration of CL(72:8) in the blood spot. Leukocyte counts were determined on a Sysmex XN‐9000 (Sysmex, Japan) automated hematology system.
Stability of blood spots in time at room temperature (RT) and 4°C: multiple blood spots were made of a single control and BTHS individual and stored without exposure to light in a desiccator with silica gel (relative humidity was around 35%) at RT (18°C‐24°C) or 4°C. The MLCL/CL ratio was measured periodically over a period of 2 years and the diagnostic performance and MLCL/CL was evaluated.
2.3. Evaluation of the diagnostic yield of 2006‐2021
MLCL/CL ratios determined in blood spots between 2006 and 2021 were collected from our laboratory information system by an automated query. There were two main sources: (1) individuals suspected of BTHS based on their clinical symptoms and/or by the presence of variants in the TAFAZZIN gene and (2) routine metabolic screening performed in our laboratory of individuals with one or more BTHS symptoms. About 60% of the requested BTHS screenings were from the first source, sent to us from all over the world. All MLCL/CL ratios that were listed as <0.01 were set at 0.01. Cases with a ratio below 0.2 were classified as non‐BTHS. Cases with a MLCL/CL ratio above 0.2 were evaluated to determine whether these were indeed BTHS or not. This was based on communications with the treating physicians concerning, clinical symptoms, results from DNA analyses, results from other biomarkers for BTHS (3‐methylglutaconic acid), the blood spot CL spectrum profile, and analyses of (ML)CL in other matrices (fibroblasts, lymphocytes) of the same individual.
3. RESULTS
Figure 1 shows extracted ion chromatograms of MLCL(52:2), CL(56:0) (the internal standard), and CL(72:8) for control and BTHS where for the latter CL(72:8) concentration is low and MLCL(52:2) accumulates whereas the inverse is true for control. Figure 2 shows the performance of the new assay with respect to its intra‐assay, inter‐assay and instrument precision (Figure 2A). The inter‐assay precision is adequate for confirmed BTHS samples, but poor for controls, primarily due to the fact that control samples have very low to undetectable MLCL levels. The intra‐assay‐precision and instrument precision are considerably better, even at low ratios. Linearity was tested in blood spots by mixing control and BTHS blood spots in different proportions. This demonstrates that CL(72:8) and MLCL(52:2) can be measured in a linear range up to at least 125 and 90 nmol/L, respectively (Figure 2B). In our clinical validation using blood spots of 63 control and 34 confirmed individuals with BTHS, concentrations of CL(72:8) in anonymized controls spanned a broader range (66‐630 nmol/L) while MLCL(52:2) was consistently low or absent, spanning a range from not detectable to 4.5 nmol/L. In BTHS, MLCL(52:2) was elevated with a minimum concentration of 24 nmol/L to a maximum of 280 nmol/L whereas CL(72:8) levels were invariably low (range 1.5‐11 nmol/L) (Figure 2C). Despite (ML)CL concentrations exceeding those tested in the linearity experiment, the MLCL/CL ratio could easily distinguish control and BTHS with a fold‐change of >2000 when comparing the median of control and BTHS (about 50‐fold for both CL(72:8) and MLCL(52:2)). We reasoned that the broad range in the CL(72:8) concentration in controls is likely dependent on the proportion of (mitochondria‐containing) leukocytes in the blood spot. Therefore, we further investigated this by correlating CL(72:8) to the leukocyte count in the same blood sample prior to making the blood spot. We indeed confirmed the expected correlation (Figure 2D), providing an explanation for the variable CL(72:8) levels in controls. During our validation experiments, we also tested storage conditions of blood spots from a control and a BTHS individual which showed that under moist‐free conditions at either room or at 4°C, a blood spot card can be stored for at least 2 years without affecting the diagnostic conclusion (despite the variation of the MLCL/CL ratio); and that when stored at room temperature it is safe to use the blood spot card for diagnostic purposes for at least 1 year (Figure 2E). Long exposure to sunlight and or moist condition can certainly affect the MLCL/CL ratio, thus influencing diagnostic parameters, and should be avoided.
FIGURE 1.
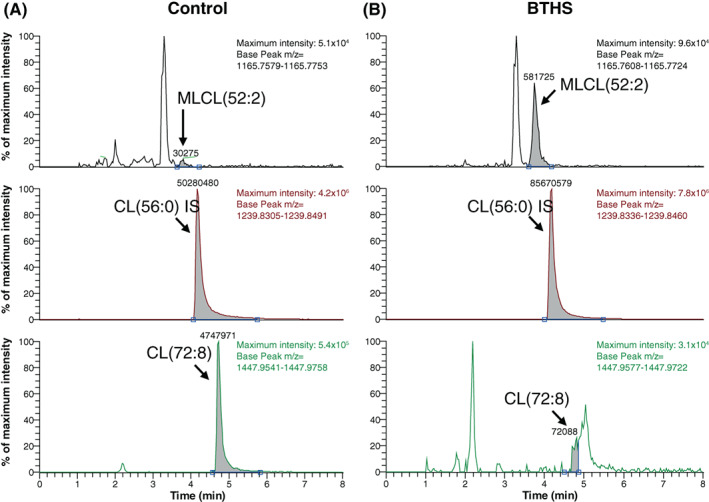
Extracted ion chromatograms of control and BTHS. Extracted ion chromatograms of MLCL(52:2), CL(56:0) = internal standard and CL(72:8) of control (left panels) and BTHS (right panel). Maximum intensity and selected m/z value (base peak) is shown in the right corner of each extracted ion chromatogram. In BTHS, MLCL(52:2) is elevated and CL(72:8) is deficient when compared to control (note the low indicated maximum intensity of the extracted ion chromatogram of the BTHS individual, about 10‐fold lower than that of the control)
FIGURE 2.
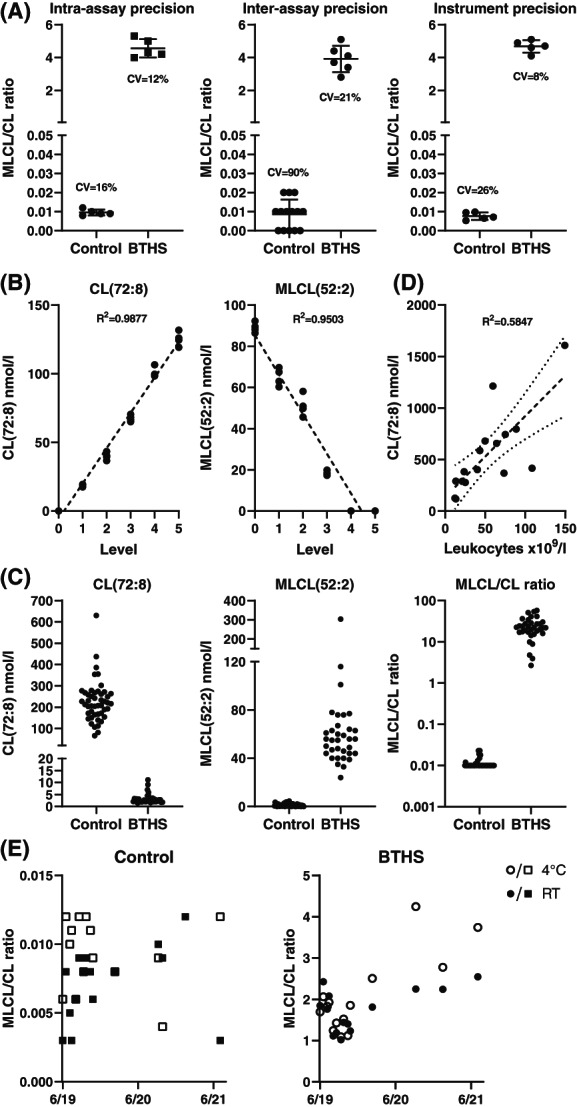
Validation of the MLCL/CL assay. (A) Intra‐assay, inter‐assay and instrument precision, CV% is indicated in the respective dot‐plots. (B) Linearity of CL(72:8) and MLCL(52:2) in blood spot by cross dilution using control and BTHS blood spots. Each level is measured n = 5 and the dotted line is the result of a linear regression analysis, correlation coefficient (R 2) is shown. (C) Clinical validation; analysis of blood spots of controls (n = 63) and BTHS (n = 34) showing dot plots for CL(72:8) and MLCL(52:2) concentrations and the MLCL/CL ratio [latter: 10log scale]. (D) Correlation analysis of CL(72:8) and the amount of leukocytes in blood spot. The dotted line is the result of a linear regression, showing the corresponding 95% confidence bands of the best fit line (small‐dotted lines) and the correlation coefficient (R 2). (E) Stability of MLCL/CL ratio of a control and BTHS individual at room temperature (RT) and 4°C over a period of 2 years. Despite variation of the MLCL/CL ratio, the control remains well below the cut‐off and the BTHS is consistently elevated in the BTHS range (>0.3)
To further enhance the diagnostic certainty, especially when the ratio is higher than 0.2, we introduced a second sample injection and corresponding analysis of the same sample to generate a broader overview spectrum of the (ML)CL range in which the fatty acid composition of the (ML)CL species can be evaluated. In BTHS, the degree of saturation of the fatty acid side chain increases (ie, less double bonds), which in the spectrum corresponds to more abundant (ML)CL species with higher m/z values than the normally linoleic acid‐enriched species. Figure 3A,B shows representative examples of a control and a BTHS blood spot spectrum for the CL species (not MLCL). In Figure 3A, CL(72:8) is the most abundant peak of the dominant 72‐cluster with a high maximum intensity of 28 × 103 whereas in the BTHS spectrum (Figure 3B) the maximum intensity is about six‐fold lower and CL(72:8) is the lowest peak of the 72‐cluster that is shifted to higher m/z values corresponding to more saturated CL species. Also, other CL clusters (68, 70, and 74) are relatively more abundant than the 72 cluster and also shifted to higher m/z values, reflecting their higher degree of saturation. In some cases, the spectrum was shifted to more saturated species but with a normal MLCL/CL ratio (Figure 3C). This was more frequently observed in newborns and severely ill children. In other cases, the abundance of CL species was low with the added difficulty of higher interference of other ions/peaks, but also in these cases, because of the high mass resolution of the mass spectrometer and the chromatographic separation, the MLCL/CL ratio could be calculated and a result could be reported (Figure 3D).
FIGURE 3.
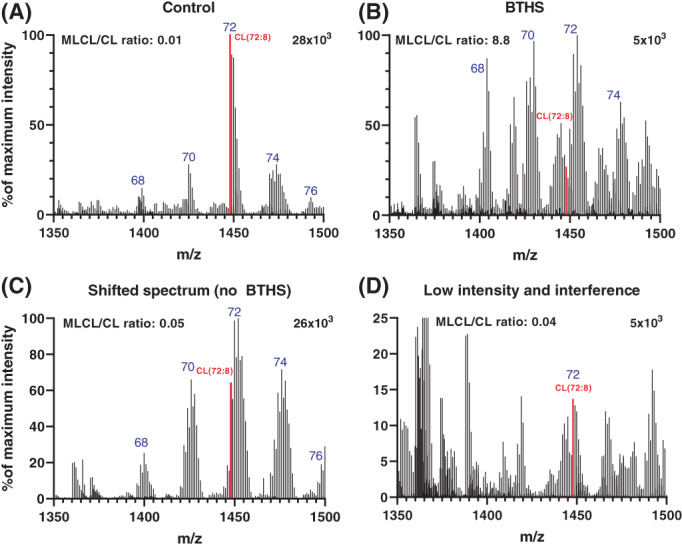
CL spectra used to evaluate a possible BTHS diagnosis. Example CL spectra of a control (A), an individual with BTHS (B), a shifted spectrum (C), and a low intensity/interference spectrum (D). Graphs show the CL range of the spectrum (m/z 1350‐1500) containing the different CL major clusters (blue, the summed amount of carbon atoms in the fatty acid side chains), the CL(72:8) peak (highlighted in red), the corresponding MLCL/CL ratio and the maximum intensity (response on the MS‐detector) corresponding to the maximum of the y‐axis shown
Having operated this assay for over 15 years, from 2006 to 2021, we decided to evaluate its diagnostic yield and performance. From 2006 to 2016, we used the original triple‐quad MS assay, and from 2016 to 2021, the improved high resolution MS assay described in this paper. We have always employed a cut‐off for the MLCL/CL ratio of 0.3, which in practice is not a hard number as some individuals with BTHS have had MLCL/CL ratios slightly below 0.3 but always had a clearly abnormal CL spectrum in blood spot, lymphocytes, or fibroblasts. During the complete period, we performed 796 blood spot measurements (662 males, 134 females) and 117 were characteristic for BTHS (15% of all test) (Figure 4). In total, considering that some of the 117 BTHS positive results were from repeat measurements, we diagnosed 93 individuals with BTHS, three of which were females, two of which have been reported previously 9 , 10 (Figure 4). This number of individuals with BTHS represents about 40% of the known 230‐250 BTHS individuals worldwide 8 and Figure 4B shows the number of individuals with diagnosed per year (on average, 6 BTHS diagnoses per year). The range of MLCL/CL ratio in individuals with BTHS was broad (0.25‐680) but did not overlap with individuals that did not have BTHS (0.01‐0.22) (Figure 4A). Especially in cases where the ratio was close to the 0.3 cut‐off we employ, the overview spectrum was crucial to come to a definitive conclusion. For some BTHS cases, we had the opportunity to measure multiple samples of the same individual collected at different occasions. The MLCL/CL ratios of these repeat analyses of the same individual showed a very broad distribution of MLCL/CL ratios (Figure 4C). The lowest BTHS was 0.25, but this was measured with the old assay (and BTHS was confirmed in lymphocytes), which is less discriminative than the current assay and analyses of other blood spots of this individual (#3 in Figure 4C)—all with the old assay‐ were all clearly in the BTHS range (0.80, 1.05, and 2.45), albeit all less elevated than the median of all individuals with BTHS (7.25).
FIGURE 4.
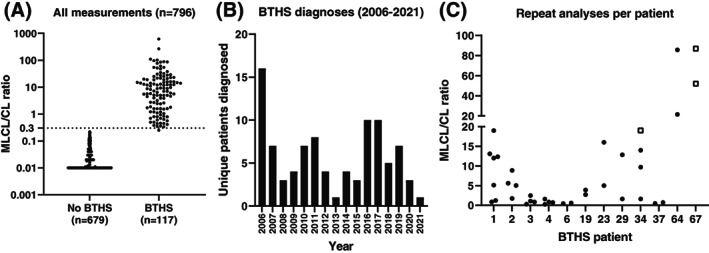
All diagnostic measurements performed from 2006 to 2021. (A) Dot‐plot of the MLCL/CL ratio of all measurements (n = 796) showing individuals selected as not having BTHS (n = 679) and those with confirmed BTHS (n = 117) [10log scale]. (B) Bar diagram of unique BTHS diagnoses per year in the period 2006‐2021. (C) MLCL/CL ratio of individuals with BTHS that underwent repeated blood spot sampling (through the years). MLCL/CL ratios are shown as black dots, but when no CL(72:8) could be detected the concentration was set to “1” and the corresponding MLCL/CL values (which are in effect infinite) are shown as white squares
4. DISCUSSION
We describe an updated and improved functional assay in blood spot that employs a ratio of two analytes, CL(72:8) and MLCL(52:2)—hereafter called the MLCL/CL ratio—to diagnose BTHS in a very accessible matrix that also is inexpensive to ship, as regular mail suffices. Several methods have been reported in literature focusing on leukocyte analysis by either MALDI‐TOF 11 or triple‐quadrupole MS 12 , 13 but, to our knowledge, no other method in blood spot has been described. Validation of the assay showed that the MLCL/CL ratio is variable and has a high inter‐assay coefficient of variation. However, considering that MLCL(52:2) is very low or even undetectable in controls and CL(72:8) in BTHS, variability is neither surprising nor worrisome. Despite this relatively high variation, the MLCL/CL ratio again proves to be an excellent biomarker that can differentiate individuals with BTHS from those not affected by this disorder. Additionally, the use of a ratio of two analytes makes precise quantification of the individual metabolites unnecessary. This is especially relevant for this assay, as specific standards of CL(72:8) and MLCL(52:2) are not available, and neither are suitable internal standards (ie, stable isotope labeled). The use of high resolution MS (as well as the transition to a newer and more advanced mass spectrometer), as employed in our new method, makes the quantification of the CL and MLCL abundance considerably better when compared to the previous triple‐quad method, 5 resulting in complete separation of control and BTHS for both compounds in the clinical validation experiment. This is most likely because only the exact mass of both compounds is selected to generate the extracted ion chromatogram, which is not possible at unit resolution on the triple quad system (where other ions can contribute to the abundance and cause more variability). Also, changing to singly‐charged (ML)CL ions in the new method (m/z range: 1165‐1447) as opposed to the previously used doubly‐charged ions (m/z range: 582‐723) moves the analysis into a m/z range that is much less crowded, further lowering the amount of background ions and thus lowering interfering signals.
The addition of the overview (ML)CL spectrum in our new method is a great asset, especially for the investigated individual/family and the clinician, as the inspection of the CL molecular species gives a conclusive BTHS/no BTHS result without the need for a confirmatory second analysis in a new blood or fibroblast sample, which is not only burdensome for the individual but also costly, time‐consuming, and labor‐intensive. In our 6‐year experience with the blood spot (ML)CL spectrum, we have seen different CL distributions that appear to depend on age, disease severity, and possibly on the diet (parenteral, enteral, type of fat source). Newborns with a normal MLCL/CL ratio frequently have a less abundant 72‐cluster and their intra‐cluster distribution is BTHS‐like, that is, shifted to more saturated species, though not as extreme as in BTHS (Figure 3C). This is possibly due to the fact that linoleic acid is an essential fatty acid that needs to be progressively gathered from the diet (via mothers milk or formula) and is not yet present in sufficient amounts to allow the linoleic acid‐enriched CL‐composition we know as normal from older individuals. Alternatively, the remodeling system in newborns may not yet be mature, similar to the glucuronic acid conjugation system that acts on bilirubin, which develops gradually after birth. 14 Investigations in mouse brain have shown that CL remodeling indeed becomes active around birth and leads to the characteristic (but different) fatty acid composition of CL in the brain, 15 thus supporting the latter option. In severely sick (non‐BTHS) individuals, we also have observed CL spectra that are shifted or contain interfering peaks (Figure 3D). This again may be caused by the source of fat from the diet, leukopenia, or medication. Despite all these aspects that may affect the CL composition of white blood cells in the blood spot, the MLCL/CL ratio remains, especially in combination with the spectrum, a robust way to investigate the possibility of BTHS.
Especially in an era where genetic diagnoses are common practice, functional testing is needed to validate the genetic findings to confirm or refute pathogenicity, particularly in case of new variants or variants of unknown significance. 16 For instance, considering that BTHS is an X‐linked recessive disorder, it is surprising that three females with BTHS have been identified. In these cases, a combination of a pathogenic variant on one allele with either chromosomal abnormalities affecting the X‐chromosome around TAFAZZIN or skewed X‐linked inactivation on the other allele caused complete dysfunction of tafazzin. 9 , 10 The functional confirmation at the biochemical level was crucial to confirm the diagnosis in these females and underscores that BTHS, although less likely, should also be considered in females with characteristic BTHS symptoms. To our knowledge, there are no cases were the MLCL/CL ratio and the ultimate clinical/genetic diagnosis was discordant, which corroborates the very high sensitivity and specificity of the assay to diagnose BTHS. In summary, the improved assay is a fast, easy, and reliable way to functionally test for BTHS in a single blood spot.
FUNDING INFORMATION
The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
CONFLICT OF INTERESTS
Riekelt Houtkooper is a consultant for Scenic Biotech and Hilary Vernon has received research and clinical trial support from Stealth BioTherapeutics in Needham, MA. All other authors have nothing to disclose.
ACKNOWLEDGMENTS
We thank all individuals with BTHS and their family for their exceptional dedication to our research. We thank the Barth syndrome foundation for their support and facilitating the collection of the precious and rare blood samples at one of the biannual Barth Syndrome Foundation (BSF) conferences.
Vaz FM, van Lenthe H, Vervaart MAT, et al. An improved functional assay in blood spot to diagnose Barth syndrome using the monolysocardiolipin/cardiolipin ratio. J Inherit Metab Dis. 2022;45(1):29‐37. doi: 10.1002/jimd.12425
Communicating Editor: Sander M Houten
REFERENCES
- 1. Vernon HJ. Clinical presentation and natural history of Barth Syndrome: An Overview. J Inherit Metab Dis. 2021. [DOI] [PubMed] [Google Scholar]
- 2. Ferreira C, Pierre G, Thompson R, Vernon H. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. Barth Syndrome. Seattle, WA: University of Washington; 1993. http://www.ncbi.nlm.nih.gov/pubmed/25299040 [PubMed] [Google Scholar]
- 3. Vreken P, Valianpour F, Nijtmans LG, et al. Defective remodeling of cardiolipin and phosphatidylglycerol in barth syndrome. Biochem Biophys Res Commun. 2000;279(2):378‐382. 10.1006/bbrc.2000.3952 [DOI] [PubMed] [Google Scholar]
- 4. Houtkooper RH, Akbari H, van Lenthe H, et al. Identification and characterization of human cardiolipin synthase. FEBS Lett. 2006;580(13):3059‐3064. 10.1016/j.febslet.2006.04.054 [DOI] [PubMed] [Google Scholar]
- 5. Kulik W, van Lenthe H, Stet FS, et al. Bloodspot assay using hplc–tandem mass spectrometry for detection of barth syndrome. Clin Chem. 2008;54:(2):371‐378. 10.1373/clinchem.2007.095711 [DOI] [PubMed] [Google Scholar]
- 6. Houtkooper RH, Rodenburg RJ, Thiels C, et al. Cardiolipin and monolysocardiolipin analysis in fibroblasts, lymphocytes, and tissues using high‐performance liquid chromatography–mass spectrometry as a diagnostic test for Barth syndrome. Anal Biochem. 2009;387(2):230‐237. 10.1016/j.ab.2009.01.032 [DOI] [PubMed] [Google Scholar]
- 7. Houtkooper RH, Turkenburg M, Poll‐The BT, et al. The enigmatic role of tafazzin in cardiolipin metabolism. Biochim Biophys Acta Biomembr. 2009;1788(10):2003‐2014. 10.1016/j.bbamem.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 8. Miller PC, Ren M, Schlame M, Toth MJ, Phoon CKL. A bayesian analysis to determine the prevalence of barth syndrome in the pediatric population. J Pediatr. 2020;217:139‐144. 10.1016/j.jpeds.2019.09.074 [DOI] [PubMed] [Google Scholar]
- 9. Cosson L, Toutain A, Simard G, et al. Barth syndrome in a female patient. Mol Genet Metab. 2012;106(1):115‐120. 10.1016/j.ymgme.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 10. Avdjieva‐Tzavella DM, Todorova AP, Kathom HM, et al. Barth syndrome in male and femal siblings caused by a novel mutation in the TAZ gene. Genet Couns. 2016;27(4):495‐501. http://www.ncbi.nlm.nih.gov/pubmed/30226969 [PubMed] [Google Scholar]
- 11. Angelini R, Lobasso S, Gorgoglione R, Bowron A, Steward CG, Corcelli A. Cardiolipin fingerprinting of leukocytes by MALDI‐TOF/MS as a screening tool for Barth syndrome. J Lipid Res. 2015;56(9):1787‐1794. 10.1194/jlr.d059824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bowron A, Honeychurch J, Williams M, et al. Barth syndrome without tetralinoleoyl cardiolipin deficiency: a possible ameliorated phenotype. J Inherit Metab Dis. 2015;38(2):279‐286. 10.1007/s10545-014-9747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowron A, Frost R, Powers VEC, Thomas PH, Heales SJR, Steward CG. Diagnosis of Barth syndrome using a novel LC‐MS/MS method for leukocyte cardiolipin analysis. J Inherit Metab Dis. 2013;36(5):741‐746. 10.1007/s10545-012-9552-4 [DOI] [PubMed] [Google Scholar]
- 14. Neumann E, Mehboob H, Ramírez J, Mirkov S, Zhang M, Liu W. Age‐dependent hepatic UDP‐glucuronosyltransferase gene expression and activity in children. Front Pharmacol. 2016;7. 10.3389/fphar.2016.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng H, Mancuso DJ, Jiang X, et al. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47(21):5869‐5880. 10.1021/bi7023282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wanders RJA, Vaz FM, Ferdinandusse S, et al. Translational Metabolism: A multidisciplinary approach towards precision diagnosis of inborn errors of metabolism in the omics era. J Inherit Metab Dis. 2019;42(2):197‐208. 10.1002/jimd.12008 [DOI] [PubMed] [Google Scholar]


