Figure 3.
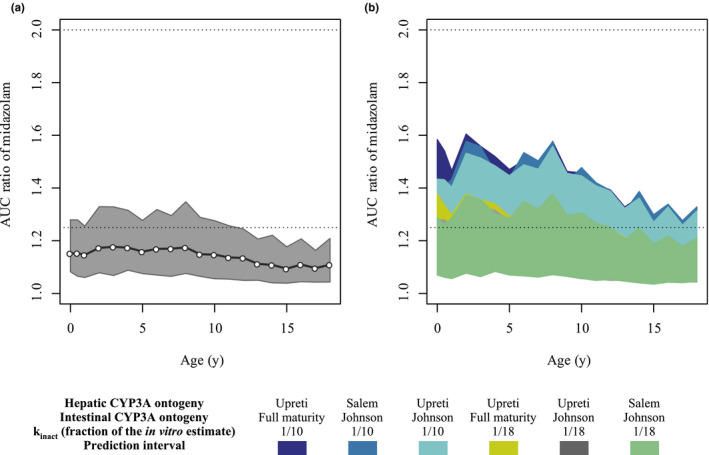
Predicted AUCR of midazolam in the presence of risdiplam in pediatric patients with SMA aged 2 months–18 years. (a) Extrapolation of the DDI using Upreti and Johnson functions for the hepatic and intestinal CYP3A ontogeny, respectively. The open circles and shaded area show geometric means and 5th to 95th percentiles of the predicted midazolam AUCR. The dotted lines indicate the ratios of 1.25 and 2 for weak and moderate inhibition, respectively. (b) Simulations with ranges of ontogeny functions and estimated in vivo k inact values to cover uncertainty in the physiology and drug‐related parameters of the DDI risk assessments. The 5th to 95th percentiles of the predicted AUCR are shown for each scenario. For UGT1A4 of the midazolam model, the steep ontogeny function for UGT1A4 (Supplementary Material ‐9 ) was used. AUCR, ratio of midazolam area under the curve with and without risdiplam; CYP, cytochrome P450; DDI, drug–drug interaction; k inact, inactivation constant; SMA, spinal muscular atrophy; UGT, uridine 5′‐diphospho‐glucuronosyltransferase.
