FIGURE 1.
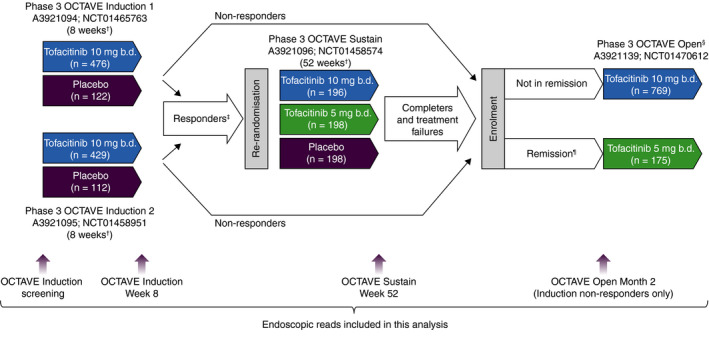
Overview of the tofacitinib phase 3 OCTAVE clinical programme. b.d., twice daily; n, number of patients treated. †Final complete efficacy assessment at Week 8/52. Treatment continued up to Week 9/53. ‡Clinical response in OCTAVE Induction 1 and 2 was defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1. §Study A3921139 (OCTAVE Open) was ongoing at the time of this interim analysis. ¶Remission was defined as a total Mayo score of ≤2 with no individual subscore >1, and a rectal bleeding subscore of 0. Adapted from Winthrop KL, et al. Inflamm Bowel Dis 2018;24:2258‐2265 (in accordance with the CC BY‐NC licence)
