FIGURE 3.
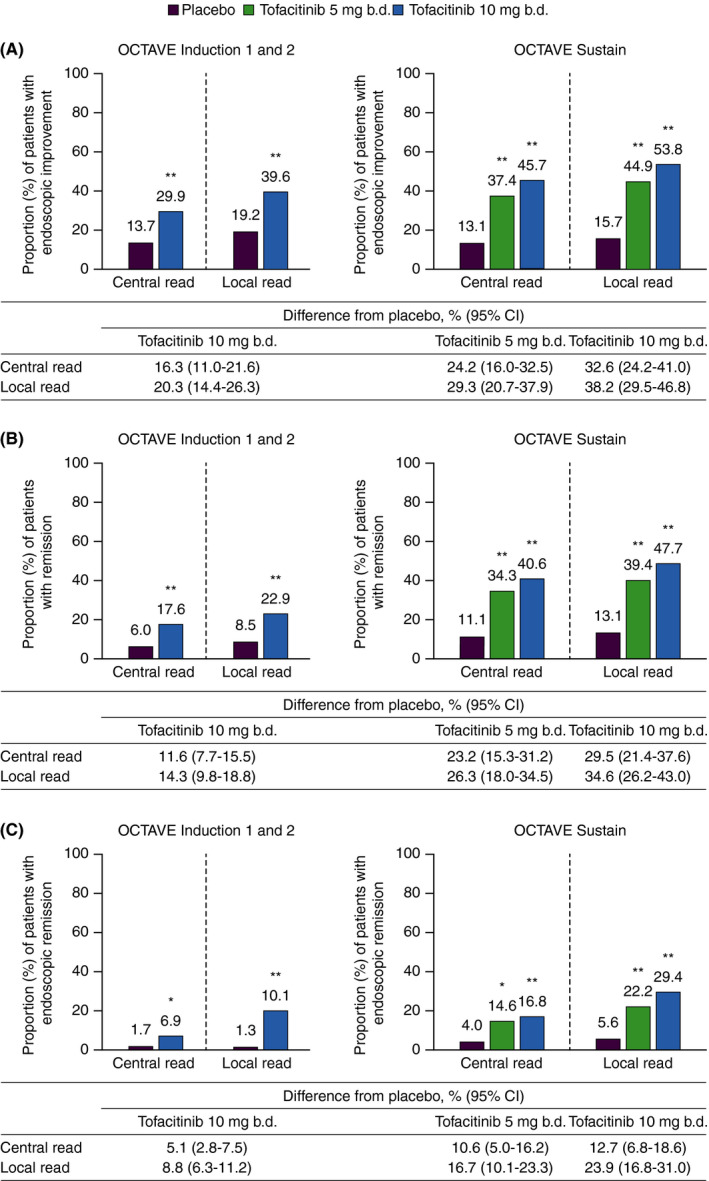
(A) Endoscopic improvement,† (B) remission‡ and (C) endoscopic remission§ at Week 8 (OCTAVE Induction 1 and 2) and Week 52 (OCTAVE Sustain), based on centrally and locally read MES. b.d., twice daily; CI, confidence interval; MES, Mayo endoscopic subscore; TNF, tumour necrosis factor. Data are full analysis set with non‐responder imputation; treatment difference from placebo is presented with 95% CIs. * P < 0.01 vs placebo. ** P < 0.001 vs placebo. For OCTAVE Induction 1 and 2, P‐values were based on the Cochran‐Mantel‐Haenszel chi‐squared test, stratified by study, prior TNF antagonist treatment, corticosteroid use at baseline and geographical region. For OCTAVE Sustain, P‐values were based on the Cochran‐Mantel‐Haenszel chi‐squared test stratified by treatment assignment in the induction study and remission at baseline. †Endoscopic improvement was defined by a MES ≤1. ‡Remission (primary endpoint) was defined as a total Mayo score ≤2 with no subscore >1, and a rectal bleeding subscore of 0. §Endoscopic remission was defined as a MES of 0
