Figure 4.
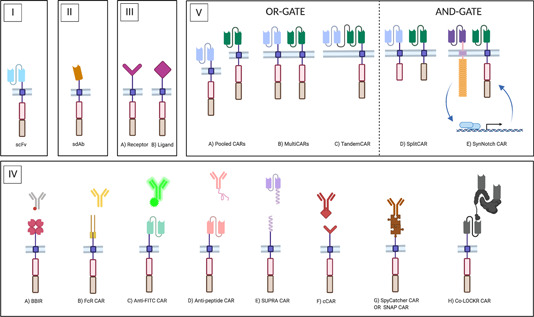
Different antigen‐binding moieties in CARs. CAR endodomains are schematically represented by a costimulatory domain (red box) and a T cell activation domain (brown box). The CAR ectodomain comprises the hinge region and the antigen‐binding moiety, which differs according to the configuration. (I) Classical CAR molecule. Antigen binding is provided by a monoclonal antibody (mAb)‐derived scFv. (II) NanoCAR. Antigen binding is provided by a sdAb, either a VHH (derived from camelid heavy‐chain‐only antibodies, HCAbs), or a VH or VL domain from the human repertoire. (III) Receptor‐ligand CARs. Either the receptor (III.A) or the ligand (III.B) portion of a naturally associating receptor‐ligand pair is incorporated as the targeting moiety. (IV) uCARs. In the uCAR configuration, the targeting domain is disconnected from the CAR module. The in vivo re‐association that must lead to CAR‐T cell engagement can take place by different types of binding: (IV.A) In the biotin‐binding immune receptor (BBIR), the CAR encodes an avidin motif which can associate with high affinity with biotinylated targeting molecules; (IV.B) FcR‐based CARs encode a FcγRIII ectodomain that can associate with the Fc‐portion of IgG‐type mAbs, thus resulting in an engineered form of antibody‐dependent cellular cytotoxicity (ADCC); (IV.C) Classical CARs with an scFv that has specificity for a FITC‐tag or a peptide tag (IV.D) on targeting molecules; (IV.E) SUPRA CARs have an extracellular leucine zipper that zips in with a complementary zipper on targeting molecules. The zipper affinity is tunable; (IV.F) Convertible CARs (cCARs) are based on a natural receptor‐ligand interaction. The CAR incorporates a variant of the NKG2D receptor that can associate with a ligand‐derivative, which is conjugated to the tracer molecule; (IV.G) SpyTag/SpyCatcher CARs and SNAP CARs rely on the formation of a covalent bond between the CAR and the adaptor molecule, via a chemical and an enzymatic reaction, respectively; (IV.H) The colocalization‐dependent protein (co‐LOCKR) CAR system consists of an anti‐tag CAR, a tagged adaptor module and a second untagged adaptor molecule that influences the conformational availability of the tag to the CAR, thereby generation AND‐, OR‐, and NOT‐gate possibilities; (V) CARs targeting more than one antigen. Left: OR‐gate CARs are designed to reduce the risk of antigen escape and to tackle heterogeneous tumors, as they only require one targeted antigen to be expressed. These include pooled CAR‐T cell populations (Pooled CARs, V.A), multiple CARs expressed by a single cell population (MultiCARs, V.B), and TandemCARs in which a single CAR molecule encodes multiple scFvs (V.C). Right: AND‐gate CARs provide increased safety, as they are active only against tumors expressing both targeted TAAs and spare healthy tissues with a single TAA expression pattern. These include (V.D) SplitCARs in which the costimulatory domain is split from the T cell activation domain, in a MultiCAR set‐up and (V.E) SynNotch CARs, in which expression of a classical CAR molecule is under control of TAAbinding of a synthetic Notch receptor that recognizes a different TAA. For simplicity reasons, the CAR moieties in (V.) are depicted with an scFv as the targeting moiety, but variations are possible. SUPRA, split, universal, and programmable [Color figure can be viewed at wileyonlinelibrary.com]
