Abstract
Aim
This study aimed to evaluate changes in prenatal testing among women with twin pregnancies before and after the introduction of noninvasive prenatal testing (NIPT). To date, no consensus on prenatal testing for twin pregnancies has been reached in Japan.
Methods
Women pregnant with twins who requested prenatal testing at Kyushu Medical Center from 2005 to 2018 were included in this study. Genetic counseling was provided to all participants. Their chosen methods of testing were collected and classified as invasive diagnosis (ID), noninvasive screening (NIS), and no test requested (NR). Parity, chorionicity, and methods of conception were assessed as attributes. The study period was divided into three terms according to testing availability in our center.
Results
After NIPT was introduced in our center, the use of ID methods decreased and eventually disappeared while NIS came to the forefront. NR was also the preferred choice of women with twin pregnancies before the introduction of NIPT and decreased but did not disappear after introducing NIPT. Women with twin pregnancies who underwent assisted reproduction initially showed hesitation to undergo testing but showed a strong preference for NIS after the introduction of NIPT. Differences in choice according to parity, chorionicity, and methods of conception were found before the introduction of NIPT but disappeared after introducing NIPT.
Conclusion
Increasing information about NIPT has apparently influenced the attitudes of women with twin pregnancies to prenatal testing in Japan. In particular, those who conceive through assisted reproductive technologies exhibited a strong preference for NIPT.
Keywords: 2.213 genetic counseling, 2.313 serum screening for aneuploidy and anomalies, 2.317 genetic amniocentesis, 2.512 multiple gestation, 4.125 assisted reproductive technology, clinical
Introduction
The increase in twin pregnancies is thought to be due to the increased use of assisted reproductive technologies (ART) and delayed childbearing, 1 , 2 , 3 which also contribute to the increasing incidence of fetal aneuploidy. Prenatal testing for fetal aneuploidy in twin pregnancies is more complex than in singleton pregnancies. Increased risk of fetal loss is associated with invasive diagnostic methods, and lower accuracy is a concern related to screening tests. 4 , 5 , 6 , 7 , 8 , 9
In Japan, from 1998 to 2008, prenatal diagnosis was performed mainly by amniocentesis (AC), but the prevalence of chorionic villous sampling (CVS) was very low. 10 The main mode of prenatal screening was maternal serum marker screening (MSM), although it was not a routine procedure. Prior to 2013, screening by ultrasonography, including nuchal translucency (NT) scanning, first‐trimester screen (FTS), or a combined test, was not predominant except in a limited number of centers. 10 , 11 This was partly due to the suggestions in “Opinions concerning tests using maternal serum markers,” a document published by the Committee on Prenatal Diagnosis at the Advanced Clinical Technology Evaluation Task Force of the Ministry of Health and Welfare (1999). The Committee stated that physicians should not give information about or recommend MSM to pregnant women because the capacity for genetic counseling (GC) was insufficient. 12 Nevertheless, there were a few centers with GC facilities that provided MSM in Japan. 13 , 14 There is still no consensus on prenatal testing for twin pregnancies in Japan.
Noninvasive prenatal testing (NIPT) using cell‐free DNA in maternal plasma has dramatically improved testing accuracy and caused major changes in prenatal testing for a singleton pregnancies. 15 This method is expected to be effective in twin pregnancies as well. 16 , 17 , 18 , 19 , 20 In 2013, NIPT was introduced in Japan, and at approximately the same time, the importance of GC prior to prenatal testing became a well‐recognized and common practice in large medical centers. 21 , 22 Takeda et al. reported the effectiveness of NIPT for twin pregnancies in Japan. 23 However, there are no reports on the attitudes of women with twin pregnancies toward prenatal testing before the introduction of NIPT.
Kyushu Medical Center has provided pretest GC prior to prenatal testing for fetal aneuploidy since 2001. In addition, as a participant in the Japan NIPT Consortium, it was one of the pioneer medical centers in the clinical research of NIPT. This study aimed to evaluate changes in the attitudes of women with twin pregnancies toward prenatal testing before and after the introduction of NIPT in Japan.
Methods
The participants included in this study were women with twin pregnancies who underwent GC for prenatal testing for fetal aneuploidy at Kyushu Medical Center from January 2005 to December 2018. Participants with vanishing twins were excluded from the study. GC for aneuploidy testing was performed by GC providers, who were either clinical geneticists certified by the Japanese Board of Medical Genetics and Genomics or genetic counselors certified by the Japanese Board of Genetic Counseling. All GC sessions were recorded whether the tests were performed or not. GC included giving women information about AC, CVS, MSM, and soft‐marker evaluation by ultrasonography. Information concerning NIPT was also included in such sessions from 2017 when our center started offering NIPT for twin pregnancies. Moreover, information regarding the safety and accuracy of each test was provided. Ethical and social concerns, including artificial abortion and prohibitions by Japanese laws, were also discussed with the women.
We reviewed the participants' records and collected information about parity (nulliparous vs. multiparous), chorionicity (monochorionic diamniotic [MD] vs. dichorionic diamniotic [DD]), methods of conception (natural conception vs. use of ART), maternal age (<35 years vs. advanced maternal age [AMA]), and gestational age at GC. Couples were divided into three groups based on their choice of test: invasive diagnosis (ID), noninvasive screening (NIS), and no test requested (NR). In our study, ID included CVS and AC. NIS included MSM and ultrasound (US) evaluations; NIPT was added to NIS from 2017. The chorionicity of all pregnancies was diagnosed by skilled obstetricians with ultrasonographic confirmation of the T‐sign or lambda sign. The study period was divided into three terms. The period from 2005 to 2012, before NIPT was introduced in Japan, was designated as Term A. The period from 2013 to 2016, when NIPT was used for singleton but not twin pregnancies in our center, was designated as Term B. The period from 2017 to 2018, when NIPT was also provided in our center for twin pregnancies, was designated as Term C. The provided prenatal tests were CVS, AC, MSM, and NT measurements during Terms A and B; NIPT and FTS were added during Term C.
The data were analyzed using statistical software (JMP Pro 14.2.0). Participants' choices of prenatal testing in each term were compared using Fisher's exact test. Distributions of maternal age and gestational age at GC were assessed using Student's t‐test. The attributes' independence was examined using the chi‐square test. Statistical significance was defined as a p‐value <0.05. This study was approved by the Ethics committee of Kyushu Medical Center (20C141). Informed consent was obtained from all participants, and the opportunity to opt‐out was provided on the home page of the center's website.
Results
Change in choices of pregnant women
During the study period (14 years), 56 women with twin pregnancies underwent GC before prenatal testing at our center. Among them, 10 women were excluded from the study due to vanishing twins, and two because AC was recommended due to their medical conditions. Forty‐four participants were finally included in the study. The number of cases in Terms A, B, and C were 13, 10, and 21, respectively. The tests selected by the participants after GC are shown in Figure 1.
FIGURE 1.
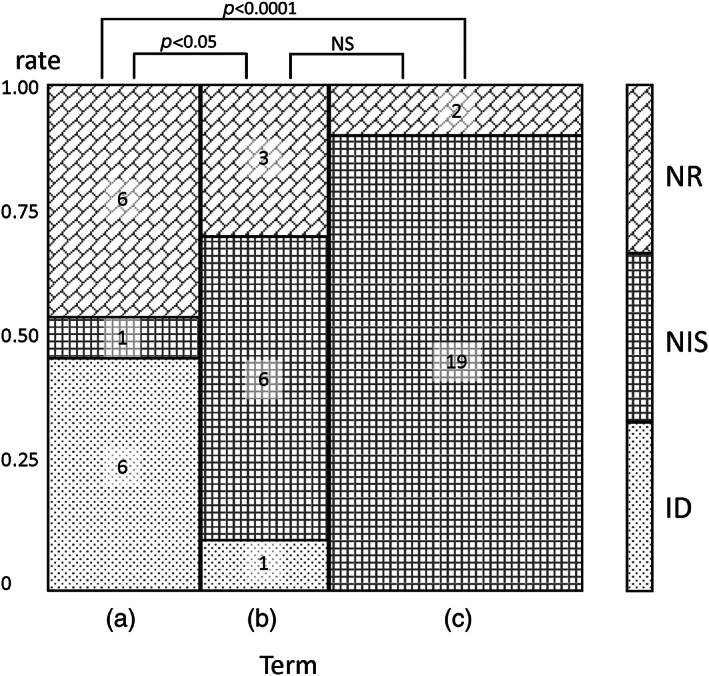
Mosaic plot of distributions of prenatal tests selected by women with twin pregnancies after GC in each term. Numbers in the columns indicate numbers of subjects. Distributions of selected prenatal tests by women with twin pregnancies were significantly different between Term A and Term B, and between Term A and Term C. Comparing NR versus “any test” (NIS + ID), a significant difference was observed only between Term A and Term C (p < 0.05). ns: GC, genetic counseling; ID, invasive diagnosis; NIS, noninvasive screening; NR, no test requested; NS, No significant difference
ID was the preferred test in Term A (46.2%). However, this number had decreased to 10% in Term B and zero in Term C. While NIS was not the preferred method for many participants in Term A (7.7%), its selection rose to 60% in Term B and 90% in Term C. The selection of NR in Term A was as high as that of ID (46.2%) but decreased slightly in Term B (30%) and sharply decreased to 9.5% in Term C. These changes in the distribution of prenatal test selection were significantly different between Terms A and B (p = 0.0271) and between Terms A and C (p < 0.0001). For reference, 28.7% of participants with singleton pregnancies in our center chose NR after GC during Term A, and 7.1% did so in Term C (data unpublished). When comparing the selection of NR with that of “any test” (NIS + ID), a significant difference was observed during Terms A and C. NIPT was only performed in Term C, during which it constituted almost all NIS cases (18/19).
Considering each term's duration (8, 4, and 2 years for Terms A, B, and C, respectively), the number of participants per year increased, most sharply in Term C (1.6, 2.5, and 10.5 participants per year in Terms A, B, and C, respectively). In contrast to the decrease in the number of times ID was selected per year (0.75, 0.25, and 0 times per year in Terms A, B, and C, respectively), an increase was observed in the number of times that NIS was selected (0.13, 1.5, and 9.5 times per year in Terms A, B, and C, respectively). NR was selected consistently (0.75, 0.75, and 1 time per year in Terms A, B, and C, respectively).
Attributes that influenced the choices of pregnant women
We subsequently compared the factors that influenced the participants' choices. We combined the participants in Terms B and C (Term B + C), and the participants choosing “any test” were grouped together. The characteristics and attributes of our participants are shown in Table 1. There was no statistically significant difference in the distribution of maternal and gestational ages at GC between Term A and Terms B + C. While there was also no significant difference when considering all attributes, associations were found among the attributes. Parity was associated with chorionicity and the method of conception; however, no association was observed between chorionicity and the method of conception. DD and ART were more likely to be observed in nulliparous than in multiparous women.
TABLE 1.
Characteristics and attributes of participants in Term A and Term B + C
| Characteristics | Term A (2005–2012) | Term B + C (2013–2016) | p‐value (Student's t‐test) |
|---|---|---|---|
| Maternal age at GC [SD] (years) | 36.4 [3.4] | 37.1 [2.6] | 0.0696 |
| Gestational age at GC [SD] (weeks + days) | 13 w 5 d [2 w 2 d] | 14 w 3 d [1 w 2 d] | 0.7139 |
| Attributes | (Fisher's exact test) | ||
| Nulliparous (%) | 8/13 (61.5) | 19/31 (61.3) | 0.6344 |
| MD twin (%) | 6/13 (46.2) | 14/31 (45.2) | 0.6534 |
| ART (%) | 5/13 (38.5) | 11/31 (35.5) | 0.7049 |
| AMA (%) | 9/13 (69.2) | 29/31 (93.5) | 0.0530 |
Abbreviations: AMA: Advanced maternal age (aged 35 years and over); ART, assisted reproductive technologies; GC, genetic counseling; MD, monochorionic diamniotic twins.
The participants' choices according to their attributes are summarized in Figure 2. In comparing the selection of participants during Term A to those during Term B + C, NR was more likely to be selected by nulliparous women (62.5% vs. 25%, p = 0.0267), those with DD pregnancies (57.1% vs. 33.3%, p = 0.0381), and those undergoing ART (80% vs. 0%, p = 0.0027) during Term A. No significant relationship was observed between the choice of test and maternal age during Term A. During Term B + C, participants tended to choose “any test” irrespective of their parity, age, chorionicity, or method of conception.
FIGURE 2.
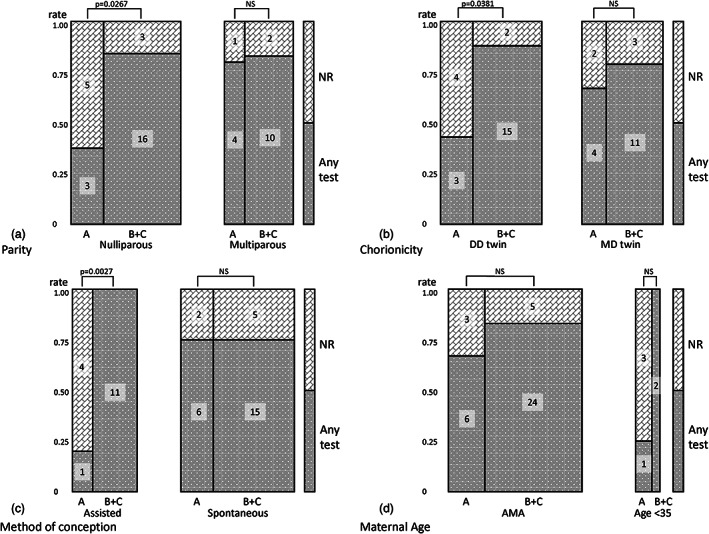
Mosaic plot of prenatal tests selected according to different attributes. Numbers in the columns indicate numbers of subjects. (a) Parity distributions of selected prenatal tests by nulliparous women are significantly different between Term A and Term B + C, while those by multiparous women are not. (b) Chorionicity distributions of selected prenatal tests by women with DD pregnancies are significantly different between Team A and Term B + C, while those by women with MD pregnancies are not. (c) Method of conception distributions of selected prenatal tests by women undergoing ART are significantly different between Term A and Term B + C, while those by women conceiving naturally are not. (d) Maternal age: no significant differences are observed in the distributions of the selected prenatal tests by women <35 years old or those of AMA. No significant differences are observed in the distributions of the selected prenatal tests, per attribute, between terms. AMA, advanced maternal age (35 years and older); ART, assisted reproductive technologies; DD, dichorionic diamniotic; MD, monochorionic diamniotic
Discussion
This study analyzed the changing attitudes to prenatal testing by women with twin pregnancies before and after NIPT was introduced. As shown in Figure 1, the participants' attitude toward prenatal testing had already changed in Term B, even before they had the choice of NIPT in Term C. Therefore, we combined Terms B and C and compared these with Term A to assess the attributes underlying this change in attitude. Because of our study's small sample size, we combined the choice of NIS with that of ID as “any test” for effective analysis.
In Figure 1, the growing demand for prenatal testing in Term C is apparent. This increase can primarily be attributed to the availability of NIPT, as all 19 tests performed in Term C were NIS, and 18 of 19 were NIPT. As the number of annual Japanese twin pregnancies were almost constant, 24 the rising demand for prenatal testing was not due to an increasing number of twin pregnancies. Rather, it was due to an increased proportion of women with twin pregnancies who requested prenatal testing. Hasegawa et al. reported an increase in pregnant patients who underwent prenatal tests from 16.2% to 24.0% following the introduction of NIPT, 25 and our results confirmed that trend.
From Term A to Term B, women with twin pregnancies exhibited an apparent change in preference in their choice of prenatal testing. One of the reasons for this change might have been the influence of the social environment. In 1999, the Committee on Prenatal Diagnosis in Japan recommended that information given to patients regarding prenatal testing be restricted 12 ; this corresponds to Term A in this study. However, in Term B, in this study, when NIPT became better known, there was an abundance of news and commentaries discussing its details, and NIPT itself was launched via mass media. The flood of information about NIPT may have evoked the population's interest and resulted in their improved literacy about prenatal testing. 21 , 26 This may have changed pregnant women's preference regarding the mode of prenatal testing from ID to NIS, especially NIPT.
The selection of NR decreased consistently throughout the study period. However, the rate of selection of NR was still high among women with twin pregnancies compared with those with singleton pregnancies, although the difference became smaller over time (46.2% vs. 28.7% in Term A, and 9.5% vs. 7.1% in Term C). This phenomenon may indicate the greater prudence of women with twin pregnancies in decision‐making about prenatal testing. In addition, this change may indicate that women with twin pregnancies became as confident as those with singleton pregnancies in Term C. Nevertheless, when considering the duration of each period, we noticed that the number of times NR was selected per year in each term remained constant (0.75–1.0 per year). The reason those participants chose not to undergo prenatal testing was not apparent in this study. However, this observation is consistent with the paradigm of nondirective pretest GC and autonomic decision‐making of pregnant women, especially those with twin pregnancies, despite the availability of NIPT.
We expected to observe more older mothers in Term B + C than in Term A because of the exclusive application of NIPT for delayed pregnancy, as recommended in Japan. 26 , 27 However, as summarized in Table 1, the distribution of maternal age at GC and AMA was not statistically significantly different between the groups. The small sample size of our study may account for this difference in the expected and observed results. This result may also reflect the fact that the indications for prenatal testing were already limited to older mothers in our institution in Term A.
The attribute that most affected the choices by women pregnant with twins between Term A and Term B + C was the “method of conception.” NR was observed as a more common choice in the ART than in the natural conception groups (80.0% and 25.0%, respectively) in Term A; however, this association was reversed in Term B + C (0% and 25.0%, respectively). It was noticeable that women with twin pregnancies after ART did not prefer prenatal testing in Term A; however, in Term B + C, they all sought prenatal screening (p = 0.0027). Similarly, Gjerris et al. reported that women becoming pregnant after ART wish to avoid invasive testing and would benefit from a prenatal screening test with high performance. 28 In contrast, it was notable that the attitude toward prenatal testing shown by women conceiving naturally did not change after the introduction of NIPT. Thus, the introduction of NIPT may have had a larger influence on Japanese women with twin pregnancies who underwent ART, in increasing their preference for NIS (NIPT in almost all cases), than on those conceiving naturally.
Similarly, nulliparous women and those with DD twins preferred NIS after introducing NIPT in Japan. Using univariate analysis, we found an association between being nulliparous and having DD twins and between being nulliparous and undergoing ART; however, our sample size was too small for multivariate analysis. Thus, the change in attitude toward prenatal testing observed in nulliparous women might have been influenced by their use of ART or having DD twins. Multicenter, large‐scale studies will be needed to clarify these associations.
From our observations, the flood of information and improved literacy evoked by the introduction of NIPT in Japan seemed to influence participants who underwent ART and those with DD twin pregnancies more than it did those who had conceived spontaneously and those with MD pregnancies. Moreover, differences in preference for prenatal testing were observed depending on parity, chorionicity, or method of conception in Term A, while these differences diminished and even disappeared in Term B + C. This might reflect the unique nature of NIPT, which is categorized as a NIS test but has high accuracy compared to conventional screening tests. Larger‐scale and multicenter studies and analyses are needed to confirm the preferences for prenatal testing of women with twin pregnancies.
Some of our results differed from those in previous reports in other Western countries. Before the introduction of NIPT, Peters et al. reported that 40% of clients with multiple gestation pregnancies declined prenatal testing/screening. 29 Our results in Term A revealed a similar NR rate (46.2%). After the introduction of NIPT, Reese et al. reported that a similar proportion of their patients (37%) declined all prenatal tests. 30 However, the NR rate in our evaluation was markedly lower (9.5%) after the introduction of NIPT. One possible explanation for this notable discrepancy may be the differences in obstetric practices for prenatal testing between Japan and the United States. Obstetricians in the United States may be more likely to educate pregnant patients on prenatal testing options. However, in Japan, obstetricians are not obligated to offer GC for prenatal testing. Therefore, their patients would need to seek out the information for themselves. Our study population did not consist merely of pregnant women, but specifically those interested in prenatal testing. Another possible reason for the decrease in NR after the introduction of NIPT in Japan may be the previous lack of experience with prenatal testing. In Japan, unlike in other industrialized countries, a statement from the Committee on Prenatal Diagnosis in 1999 restricted physicians from actively informing pregnant women about MSM.12 Therefore, knowledge about prenatal testing had been poor among the general population in Japan and subsequently increased dramatically with the introduction of NIPT; for many women, it may have appeared that NIPT was the first available prenatal test.
In addition, unlike the increase in the utilization of ID in nulliparous women reported by Halliday et al., 31 nulliparous women exhibited a greater preference for NR than multiparous women in our investigation. The association between nulliparity and ART observed in our study might have contributed to this difference because women who become pregnant after ART tend to avoid ID tests. 26 Another explanation for this discrepancy may be differences in the study populations, such as the number of participants or the difference in birth rates between the study populations.
Two factors may have influenced the choice of prenatal tests by women with twin pregnancies in our study. The age‐related risk for having an aneuploid fetus is higher in women pregnant with dizygotic twins than in those with singleton pregnancies. In the literature, a range of maternal ages (31–33 years) has been suggested for offering invasive prenatal diagnosis to women with twin pregnancies. 4 , 32 In contrast, several reports have stated that the increased risk of aneuploidy in twin pregnancies is not as high as was expected. Boyle et al. reported that the relative risk of having a fetus with trisomy 21 in a pregnancy with monozygotic twins versus a singleton pregnancy was 0.34, while that of a dizygotic twin pregnancy versus a singleton pregnancy was 1.34. 33 Sparks et al. reported a similar observation. 34 In this study, our GC was based on the former point of view. Therefore, the estimated risk conveyed during GC sessions in this study may have been higher than warranted by recent results. Another factor was the cost of prenatal testing. The cost of each test did not differ substantially across Terms A, B, and C. However, in Term C, ID after a positive NIPT result became free in Japan, as clinical research. This may have motivated women with twin pregnancies to choose NIPT.
The limitations of this study are similar to those of other long‐term studies, in that we did not consider the influences of major societal changes (such as welfare policies or the societal attitude toward eugenics). In addition, the small number of participants available at a single facility precludes meaningful multivariable analysis. A further limitation is that we confined our study to prenatal testing performed at our own institution.
In conclusion, although this was a preliminary single‐center study, it appears that the introduction of NIPT influenced the attitudes of women pregnant with twins toward prenatal testing in Japan. This is especially true of those who conceived by ART. Interestingly, this change may have arisen because of the spread of information about NIPT, even prior to its actual implementation in Japan. Pretest GC is essential for women pregnant with twins to make an informed choice regarding prenatal tests.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgment
The authors thank Prof. Mototsugu Shimokawa for guidance in the statistical analyses.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. McNamara HC, Kane SC, Craig JM, et al. A review of the mechanisms and evidence for typical and atypical twinning. Am J Obstet Gynecol. 2016;214:172–91. 10.1016/j.ajog.2015.10.930 [DOI] [PubMed] [Google Scholar]
- 2. Blondel B, Kaminski M. Trends in the occurrence, determinants, and consequences of multiple births. Semin Perinatol. 2002;26:239–49. 10.1053/sper.2002.34775 [DOI] [PubMed] [Google Scholar]
- 3. Cleary‐Goldman J, D'Alton ME, Berkowitz RL. Prenatal diagnosis and multiple pregnancy. Semin Perinatol. 2005;29:312–20. 10.1053/j.semperi.2005.08.005 [DOI] [PubMed] [Google Scholar]
- 4. Wapner RJ. Genetic diagnosis in multiple pregnancies. Semin Perinatol. 1995;19:351–62. 10.1016/s0146-0005(05)80013-8 [DOI] [PubMed] [Google Scholar]
- 5. Drugan A, O'Brien JE, Dvorin E, et al. Multiple marker screening in multifetal gestations: failure to predict adverse pregnancy outcomes. Fetal Diagn Ther. 1996;11:16–9. 10.1159/000264273 [DOI] [PubMed] [Google Scholar]
- 6. Jenkins TM, Wapner RJ. The challenge of prenatal diagnosis in twin pregnancies. Curr Opin Obstet Gynecol. 2000;12:87–92. 10.1097/00001703-200004000-00006 [DOI] [PubMed] [Google Scholar]
- 7. Yukobowich E, Anteby EY, Cohen SM, Lavy Y, Granat M, Yagel S. Risk of fetal loss in twin pregnancies undergoing second trimester amniocentesis. Obstet Gynecol. 2001;98:231–4. 10.1016/s0029-7844(01)01416-8 [DOI] [PubMed] [Google Scholar]
- 8. Maymon R, Neeman O, Shulman A, Rosen H, Herman A. Current concepts of down syndrome screening tests in assisted reproduction twin pregnancies: another double trouble. Prenat Diagn. 2005;25:746–50. 10.1002/pd.1259 [DOI] [PubMed] [Google Scholar]
- 9. Bush MC, Malone FD. Down syndrome screening in twins. Clin Perinatol. 2005;32:373–86. 10.1016/j.clp.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 10. Sasaki A, Sawai H, Masuzaki M, Hirahara F, Sago H. Low prevalence of genetic prenatal diagnosis in Japan. Prenat Diagn. 2011;31:1007–9. 10.1002/pd.2816 [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama M, Yan J, Yotsumoto J, Sawai H, Sekizawa A, Kamei Y, et al. Chromosome abnormalities diagnosed in utero: a Japanese study of 28983 amniotic fluid specimens collected before 22 weeks gestations. J Hum Genet. 2015;60:133–7. 10.1038/jhg.2014.116 [DOI] [PubMed] [Google Scholar]
- 12. ‘Opinions concerning tests using maternal serum markers.’ The Committee on Prenatal Diagnosis, the Advanced Clinical Technology Evaluation Task Force of the Ministry of Health and Welfare , 1999. Available from the Ministry of Health, Labour and Welfare, Japan website, [Accessed 1st Sept 2020.] URL: https://www.mhlw.go.jp/www1/houdou/1107/h0721-1_18.html (Japanese)
- 13. Okuyama T, Yotsumoto J, Funato Y. Survey of second‐trimester maternal serum screening in Japan. J Obstet Gynaecol Res. 2013;39:942–7. 10.1111/jog.12015 [DOI] [PubMed] [Google Scholar]
- 14. Miyake H, Yamada S, Fujii Y, Sawai H, Arimori N, Yamanouchi Y, et al. Nationwide survey for current clinical status of amniocentesis and maternal serum marker test in Japan. J Hum Genet. 2016;61:879–84. 10.1038/jhg.2016.67 [DOI] [PubMed] [Google Scholar]
- 15. Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18:1056–65. 10.1038/gim.2016.97 [DOI] [PubMed] [Google Scholar]
- 16. Tan Y, Gao Y, Lin G, Fu M, Li X, Yin X, et al. Noninvasive prenatal testing (NIPT) in twin pregnancies with treatment of assisted reproductive techniques (ART) in a single center. Prenat Diagn. 2016;36:672–9. 10.1002/pd.4837 [DOI] [PubMed] [Google Scholar]
- 17. Fosler L, Winters P, Jones KW, Curnow KJ, Sehnert AJ, Bhatt S, et al. Aneuploidy screening by non‐invasive prenatal testing in twin pregnancy. Ultrasound Obstet Gynecol. 2017;49:470–7. 10.1002/uog.15964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Conte G, Letourneau A, Jani J, et al. Cell‐free fetal DNA analysis in maternal plasma as screening test for trisomies 21, 18 and 13 in twin pregnancy. Ultrasound in Obstet Gynecol. 2018;52:318–24. 10.1002/uog.18838 [DOI] [PubMed] [Google Scholar]
- 19. Dyr B, Boomer T, Almasri EA, Wardrop JL, Rafalko J, Chibuk J, et al. A new era in aneuploidy screening: cfDNA testing in >30,000 multifetal gestations: experience at one clinical laboratory. PLoS One. 2019;14:e0220979. 10.1371/journal.pone.0220979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motevasselian M, Gargari SS, Younesi S, et al. Non‐invasive prenatal test to screen common trisomies in twin pregnancies. Mol Cytogenet. 2020;13:5. 10.1186/s13039-020-0475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yotsumoto J, Sekizawa A, Suzumori N, et al. A survey on awareness of genetic counseling for non‐invasive prenatal testing: the first year experience in Japan. J Hum Genet. 2016;61:995–1001. 10.1038/jhg.2016.96 [DOI] [PubMed] [Google Scholar]
- 22. Samura O, Sekizawa A, Suzumori N, Sasaki A, Wada S, Hamanoue H, et al. Current status of non‐invasive prenatal testing in Japan. J Obstet Gynaecou Res. 2017;43:1245–55. 10.1111/jog.13373 [DOI] [PubMed] [Google Scholar]
- 23. Takeda E, Suzumori N, Kumagai K, Inuzuka S, Oseto K, Ohigashi Y, et al. Performance and outcomes of noninvasive prenatal testing for twin pregnancies in Japan. J Obstet Gynaecol Res. 2018;44:1909–14. 10.1111/jog.13744 [DOI] [PubMed] [Google Scholar]
- 24. Vital Statistics (Ministry of Health, Labour and Welfare, Japan) . Available from ‘Portal Site of Official Statistics of Japan website,’ [Accessed 1st Sept 2020.] URL: https://www.e-stat.go.jp/
- 25. Hasegawa J, Nakamura M, Sekizawa A. How do the trends in the prenatal diagnosis of aneuploidy change after a non‐invasive prenatal test becomes available? A Japanese single center study. J Med Ultrasonics. 2015;42:195–8. 10.1007/s10396-014-0589-x [DOI] [PubMed] [Google Scholar]
- 26. Mikamo S, Nakatsuka M. Knowledge and attitudes toward non‐invasive prenatal testing among pregnant Japanese women. Acta Med. Okayama. 2015;69:155–63. 10.18926/AMO/53522 [DOI] [PubMed] [Google Scholar]
- 27. Sago H, Sekizawa A. Japan NIPT consortium. Nationwide demonstration project of next‐generation sequencing of cell‐free DNA in maternal plasma in Japan: 1‐year experience. Prenat Diagn. 2015;35:331–6. 10.1002/pd.4539 [DOI] [PubMed] [Google Scholar]
- 28. Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. Prenatal testing among women pregnant after assisted reproductive techniques in Denmark 1995‐2000: a national cohort study. Hum Reprod. 2008;23:1545–52. 10.1093/humrep/den103 [DOI] [PubMed] [Google Scholar]
- 29. Peters KF, Saltman BM, Petrill SA. Twin gestation pregnancies: genetic counseling and testing experience. J Genet Counsel. 2006;15:119–27. 10.1007/s10897-005-9007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reese KM, Czerwinski J, Darilek S, Johnson A, Jones M, Singletary CN. Attitudes toward and uptake of prenatal genetic screening and testing in twin pregnancies. J Genet Counsel. 2018;27:1238–47. 10.1007/s10897-018-0246-4 [DOI] [PubMed] [Google Scholar]
- 31. Halliday J, Lumley J, Watson L. Comparison of women who do and do not have amniocentesis or chorionic villus sampling. Lancet. 1995;345:704–9. 10.1016/S0140-6736(95)90872-2 [DOI] [PubMed] [Google Scholar]
- 32. Meyers C, Adam R, Dungan J, Prenger V. Aneuploidy in twin gestations: when is maternal age advanced? Obstet Gynecol. 1997;89:248–51. 10.1016/s0029-7844(96)00424-3 [DOI] [PubMed] [Google Scholar]
- 33. Boyle B, Morris LK, McConkey R, et al. Prevalence and risk of down syndrome in monozygotic and dizygotic multiple pregnancies in Europe: implications for prenatal screening. BJOG. 2014;121:809–20. 10.1111/1471-0528.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sparks TN, Norton ME, Flessel M, Goldman S, Currier RJ. Observed rate of down syndrome in twin pregnancies. Obstet Gynecol. 2016;128:1127–33. 10.1097/AOG.0000000000001690 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


