FIGURE 1.
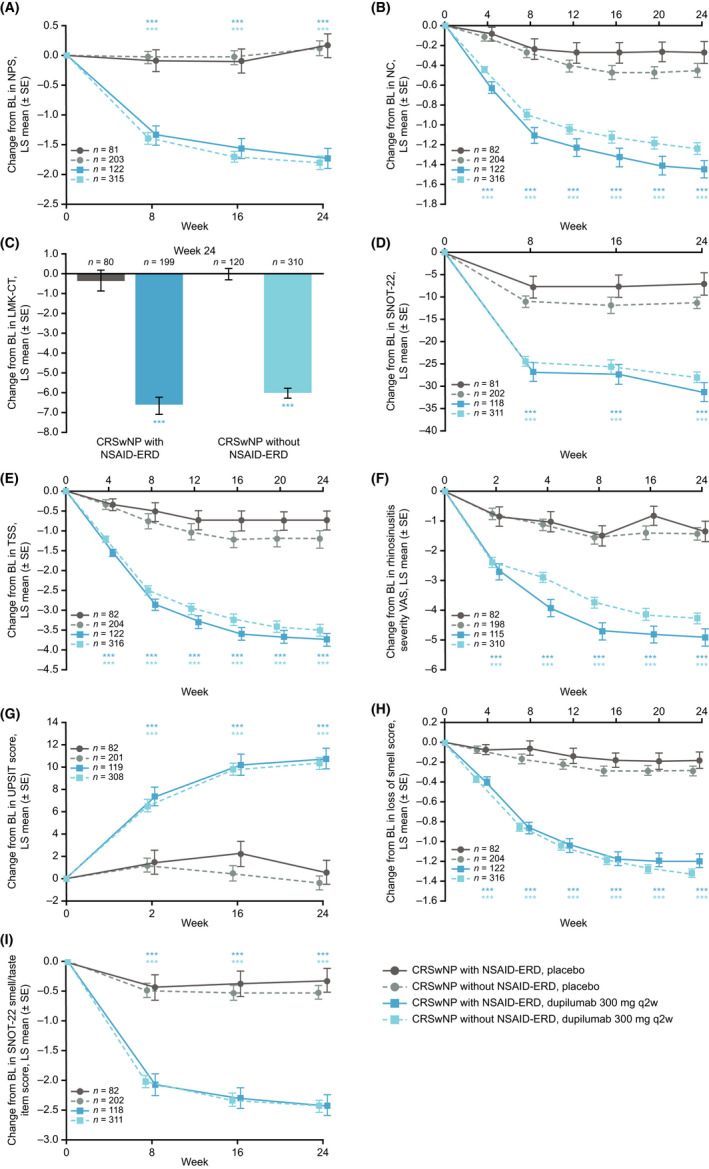
Changes from baseline to Week 24 in CRSwNP disease control and symptom burden in patients with CRSwNP with and without NSAID‐ERD, as assessed by (A) NPS, (B) patient‐assessed symptom severity score for NC or obstruction, (C) LMK‐CT score, (D) SNOT‐22 score, (E) patient‐reported TSS, (F) VAS for rhinosinusitis, (G) UPSIT score, (H) loss of smell score, and (I) SNOT‐22 smell/taste item score. ***Nominal p < .0001 versus placebo. Abbreviations: BL, baseline; CRSwNP, chronic rhinosinusitis with nasal polyps; LMK‐CT, Lund–Mackay computed tomography; LS, least squares; NPS, nasal polyp score; NSAID‐ERD, non‐steroidal anti‐inflammatory drug‐exacerbated disease; q2w, every 2 weeks; SE, standard error; SNOT‐22, 22‐item Sinonasal Outcome Test; TSS, Total Symptom Score; UPSIT, University of Pennsylvania Smell Identification Test; VAS, visual analog scale.
