Figure 4.
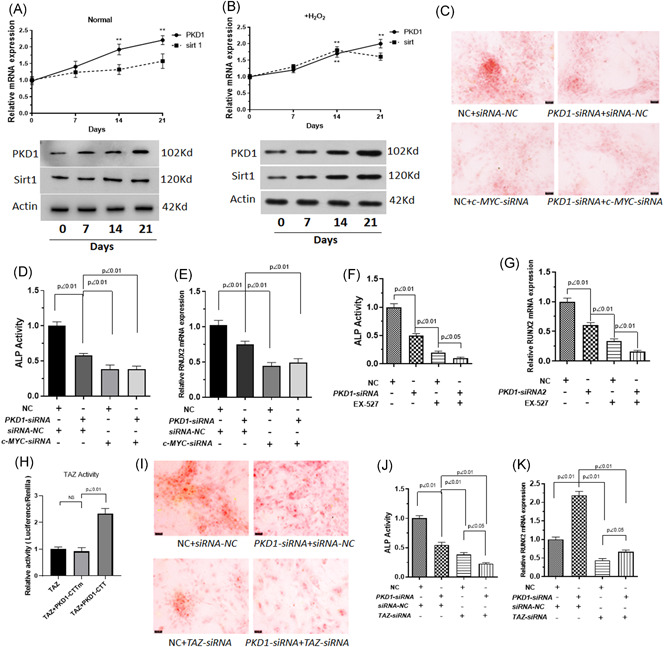
PKD1 regulates osteogenic differentiation by interacting with TAZ under oxidative stress. BM‐MSCs were pre‐exposed or not to 100 μM H2O2 diluted in DMEM for 24 h followed by incubation in differentiation medium (ODM) for 0–20 days and/or treated with the Sirt1 inhibitor, Selisistat (EX 527). Cells were transfected with PKD1‐siRNA, c‐MYC‐siRNA, or TAZ‐siRNA. (A, B) Protein and mRNA expression of PKD1 and Sirt1 was measured by western blot analysis and qPCR under normal conditions or oxidative stress. (C) Alizarin red S staining for the presence of calcium deposits was performed to investigate the effect of PKD1 combined with c‐MYC on the osteogenic differentiation of BM‐MSCs under oxidative stress. (D, F) ALP activity was measured. (E, G) qPCR assays were used to measure RUNX2 mRNA expression. (H) Dual‐luciferase reporter assays were used to evaluate the relation between PKD1 and TAZ activity. (I) Alizarin red S staining was used to detect the presence of calcium deposits, (J) ALP activity, and (K) RUNX2 mRNA expression were obtained from qPCR detection to investigate the effect of PKD1 combined with TAZ on the osteogenic differentiation of BM‐MSCs under oxidative stress. Data are expressed as the mean ± SD, n = 3. NS, not significant. BM‐MSCs, bone marrow‐mesenchymal stem cells; DMEM, Dulbecco's modified eagle medium; mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction
