Abstract
Introduction and Objectives
Surface depressions and skin laxity together play a role in the appearance of cellulite. Cellulite depressions can be improved through disruption of the subcutaneous fibrous structures. Some currently utilized approaches accomplish this through invasive techniques requiring local anesthesia and potential down time. Skin laxity can exacerbate the appearance of cellulite, however current invasive approaches do little to improve skin laxity. The objective of this study was to evaluate a noninvasive approach to improving both cellulite depressions and skin laxity through the use of rapid acoustic pulses (acoustic subcision). Safety, efficacy, tolerability, and participant satisfaction results were measured.
Methods
Women (n = 56) with moderate to severe cellulite were treated in a single acoustic subcision treatment session without anesthesia. Posttreatment adverse events (AEs) and tolerability were recorded. At 12‐weeks cellulite outcomes were assessed using a 6‐point simplified Cellulite Severity Scale (CSS), Global Aesthetic Improvement Scale (GAIS), and a participant satisfaction questionnaire. Additionally, laxity improvement was measured using a 4‐point Laxity Score (LS) and GAIS.
Results
Improvement in cellulite appearance measured at 12‐weeks showed that participants (n = 56) had a mean CSS reduction of 1.01 (a 29.5% reduction from baseline). The posttreatment photograph was correctly identified by blinded independent reviewers from randomized pairs of pre/posttreatment photographs for 96.4% of participants. Cellulite was graded as improved, much improved or very much improved using the GAIS at 90.9% of treated locations. Finally, 92.9% of participants reported positive satisfaction responses. Scoring for improvement in skin laxity appearance at 12‐weeks showed a mean LS reduction of 0.57 (a 27.9% reduction from baseline). GAIS for laxity was graded as improved, much improved or very much improved in 67.3% of treated areas. No unexpected or serious AEs were noted at treatment or follow‐up. Overall average pain score during treatment was 2.4 (0–10 pain scale) and 0.3 immediately posttreatment.
Conclusion
A single noninvasive acoustic subcision session can safely provide meaningful improvement in the appearance of cellulite in terms of depressions, as well as skin laxity, with minimal treatment pain and no posttreatment down time. Further improvement in appearance is expected with multiple treatments over time. Additional trials to verify this are planned.
Keywords: acoustic subcision, cellulite, improvement in appearance, laxity, noninvasive, rapid acoustic pulse
1. INTRODUCTION
Cellulite is an aesthetically displeasing rippling or dimpling of the skin most commonly located on the thighs and buttocks of women. Its appearance and texture are often referred to as “cottage cheese” or an “orange peel.” 1
In a manuscript discussing anatomical approaches to treating cellulite by Christman et al. 1 they state “In normal skin, there is a support network of fibrous septae running through the subcutis, separating the adipose cells into chambers resembling a quilt. Magnetic resonance imaging demonstrates that in cellulite these fibrous septae are contracted and sclerosed ultimately tethering the skin at a fixed length.” Kaminer et al. 2 demonstrated long‐lasting improvement in the appearance of cellulite through minimally invasive mechanical subcision of these septae. Cellulite severity is also influenced by skin laxity, particularly in older individuals. 3 , 4 Treating skin laxity by improving dermal strength and elasticity are potentially important elements for treatments targeting the appearance of cellulite dimples. 4 , 5 , 6
The acoustic subcision device (Soliton, Inc.) uses rapid acoustic pulses designed to improve the appearance of cellulite. The authors hypothesize that rapid acoustic pulses noninvasively disrupt the fibrous septae (i.e., subcision) in the subcutaneous extracellular matrix space, leading to tissue release that results in the improvement in the appearance of cellulite. We further hypothesize that rapid acoustic pulse's mechanical action on the dermal extracellular matrix leads to dermal neocollagenesis resulting in improvement in the appearance of skin laxity.
The acoustic subcision device as represented in Figure 1 is composed of three parts: the console, the hand piece, and a disposable cartridge. The console houses the power supply used to provide high voltage to electrodes that are housed in the cartridge which can be replaced when the electrodes wear out. Additionally, the console contains a fluid management system that circulates saline through the cartridge for cooling. The cartridge is snapped in and out of the hand piece for quick replacement. Figure 2 shows the hand piece in use during application in a cellulite treatment session.
Figure 1.

Acoustic subcision device
Figure 2.
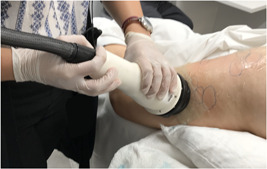
Photograph of hand piece in use during application in a cellulite treatment session
The objective of this study is to evaluate the safety, efficacy, tolerability, and participant satisfaction of a noninvasive acoustic subcision treatment for the temporary improvement in the appearance of cellulite and laxity in a multicenter clinical study.
2. MATERIALS AND METHODS
The use of the acoustic subcision device in a multicenter, prospective study had been determined to present a nonsignificant risk in accordance with 21 CFR 812.3 for the intended use in this study by the overseeing Institutional Review Board (Quorum Review IRB). The study also conformed to US Federal Policy for the Protection of Human Subjects. The study is registered on Clinicaltrails.gov as NCT04065711.
2.1. Participants
Inclusion criteria for study participants included healthy individuals between the ages of 18–50 that had severe cellulite (≥4.0) on at least one thigh and/or buttock on a 6‐point Cellulite Severity Scale (CSS) at baseline as determined by the clinical site principal investigator. Additional inclusion criteria included: Stable weight nominally ±5% for at least 6 months before the study; Body Mass Index (BMI) ≤ 30; no invasive or energy‐based cellulite treatments (liposuction, subcision, RF, laser, ESWT, etc.) for the prior 12 months; no use of topical based cellulite treatments for the prior 6 months. Key exclusion criteria included: pregnant or planning to become pregnant during the duration of the study; metal or plastic implants in the area of the treatment (vascular stent, or implants in the hips, knees, etc.); active electronic implants such as pacemakers, defibrillators, cochlear implants, nerve/brain stimulators, drug pump, and so on; a medical disorder that would hinder wound healing or immune response (no blood disorder, inflammatory disease, etc.) or coagulopathy(ies) and/or is on anticoagulant medication; skin disorders (skin infections or rashes, extensive scarring, psoriasis, etc.) in the treatment area; any surgical procedure in the prior 3 months, or planned during the duration of the study; and current smoker.
2.2. Treatment
Acoustic subcision treatment was administered during a single office session following completion of screening, enrollment, and obtaining informed consent from each participant. The investigator selected cellulite either on the right or left side buttock/leg with the most, or most serious cellulite, and marked 9–15 treatment areas (i.e., depressions, dimples, ridges, etc.). The participant was then positioned either in the lateral decubitus or prone position and an acoustic coupling hydrogel pad and hydrogel was applied to the marked treatment areas. No anesthesia or other pain medications were given to the participant before, during or following the procedure.
One to two 1‐min acoustic subcision doses were administered at a pulse rate of 50 Hz to each treatment area. The total number of treatment doses was determined by the number of identified treatment areas. Additional 1‐min doses were administered to deep dimples and ridges and areas between and around the marked treatment areas. Substantially all treatments were administered by each clinical site's nurses or technicians. Treatment areas were cleaned immediately posttreatment and evaluated for any adverse events (AEs). No posttreatment bandages, compressions garments, or other care were needed.
2.3. Photographs
Serial clinical photographs (QuantifiCare 3D LifeViz stereoscopic camera system) were collected at baseline and 12‐week posttreatment timepoints. The participants stood in a relaxed position on a rotatable platform and photographs were taken of the thighs, buttocks and the thighs and buttocks together from eight angles. Photographs were taken by each clinical site's staff under standardized conditions, including the angle of the lights and distances between the platform, lights and camera.
2.4. Assessment
Immediately following treatment, the participants were evaluated for AEs. Additionally, participants were asked to grade procedure pain and then pain after treatment on a 0–10 pain scale. Three blinded, independent, Board Certified dermatologists (Reviewers) first identified posttreatment images from randomly ordered side‐by‐side baseline and 12‐week photographs and then graded the baseline and 12‐week posttreatment images using a simplified 6‐point CSS 2 Additionally, the improvement in the appearance of cellulite was rated according to the Global Aesthetic Improvement Scale (GAIS) (i.e., Very much improved, much improved, improved, no change, or worse). In addition to assessing the improvement in the appearance of cellulite, the Reviewers scored the baseline and 12‐week posttreatment photos to assess the improvement in the appearance of leg skin laxity using a 4‐point Skin Laxity Scale (LS). This scale was based on the LS component of Hexsel's validated photonumeric CSS. 6 Reviewers also provided a GAIS score for change in skin laxity from baseline to 12‐weeks. Finally, after reviewing their own baseline and 12‐week posttreatment photographs participants were given a satisfaction questionnaire using a 5‐point scale (Strongly agree = 2; Agree = 1; Neutral = 0; Disagree = −1; Strongly disagree = −2) see Table 4.
Table 4.
Toleration and Satisfaction
| (N = 56) | |
|---|---|
| Toleration | |
| Procedure tolerability | |
| Participants stated procedure is tolerable (%) | 98.2% |
| Overall pain (0–10 pain scale with 0 = no pain) | |
| During individual treatments (Mean ± SD) | 2.4 ± 1.4 |
| 95% CI (Lower–upper) | (2.0–2.8) |
| Range (low–high) | (0–6) |
| After treatments (mean ± SD) | 0.3 ± 0.8 |
| 95% CI (Lower–upper) | (0.1–0.5) |
| Range (low–high) | (0–5) |
| Satisfaction | |
| “Cellulite appears improved” | |
| Agree and/or strongly agree | 92.9% |
| Neutral | 5.4% |
| Disagree and/or strongly disagree | 1.8% |
| “The RAP treatment was relatively pain‐free” | |
| Agree and/or strongly agree | 76.8% |
| Neutral | 14.3% |
| Disagree and/or strongly disagree | 8.9% |
2.5. End points
The primary safety end point was no reported UAEs or SAEs immediately following acoustic subcision treatment and at the 12‐week visit. The primary efficacy end point was to demonstrate a temporary improvement in the appearance of cellulite by showing a ≥ 1 point mean reduction in the simplified CSS from baseline to 12‐weeks. Secondary end points included: (1) The Reviewers correctly identifying, at an average accuracy of 60% or greater, the 12‐week posttreatment photographs from the baseline photographs; (2) There was an average improvement in cellulite appearance as measured by the GAIS; (3) There was an average improvement in the appearance of skin laxity as measured using the LS and the GAIS.
2.6. Statistical analysis
Baseline and 12‐week data were summarized as mean, SD, confidence interval, minimum, and maximum for continuous variables where appropriate. Significance testing was performed using Student's t‐test (two‐sided, paired) or χ 2 test using GraphPad Prism version 9.00.0 for Windows, GraphPad Software, www.graphpad.com.
3. RESULTS
3.1. Participants
Sixty‐seven (67) participants were enrolled in the clinical study and treated. At the 12‐week follow‐up visit, 11 participants were lost to follow‐up, exclusionary criteria or camera malfunction. Table 1 provides a breakdown of demographics for the study participant population.
Table 1.
Baseline characteristics distribution for the study participant population
| Participants | |
|---|---|
| Enrolled | |
| Totaled enrolled and treated | 67 |
| Lost | |
| Subjects lost to follow‐up, exclusionary criteria or camera malfunction | 11 |
| Evaluated | |
| Subjects evaluated at 12‐weeks | 56 |
| Gender | |
| Female | 100% |
| Race | |
| White/Caucasian | 92.9% |
| African American/Black | 3.6% |
| Asian | 3.6% |
| Ethnicity | |
| Not Hispanic/not Latino | 92.9% |
| Hispanic/Latino | 7.1% |
| Age | |
| Mean ± SD | 43 ± 6.7 |
| (min–max) | (25.7–50.8) |
| Weight (pounds) | |
| Mean ± SD | 150.0 ± 21.6 |
| (min–max) | (102.0–191.4) |
| BMI | |
| Mean ± SD | 24.5 ± 2.8 |
| (min–max) | (18.9–30.0) |
3.2. Treatment doses
The average number of delivered 1‐min doses was 28 (minimum of 19 and maximum of 34 delivered 1‐min doses).
3.3. Efficacy
3.3.1. Improvement in the appearance of cellulite depressions
As summarized in Table 2 all endpoints were met. The mean reduction in the simplified CSS was 1.01 which met the primary endpoint for overall efficacy. This represented a 29.6% reduction from the baseline CSS. The difference between the baseline and 12‐week CSS was statistically significant (p < 0.0001, t‐test, two‐tailed, paired). The Reviewers correctly identified the posttreatment photograph at a rate of 96.4% which met the secondary endpoint for overall efficacy. This successful selection rate was statistically significant (p < 0.001) based on a χ 2 test to assess the null hypothesis that the rate that the evaluators can identify the baseline photographs is greater than 50%. Finally, Reviewers rated 90.9% of the treated cellulite sites as appearing improved, much improved or very much improved using a GAIS. Representative serial photographs demonstrating improvement in the appearance of cellulite are shown in Figures 3, 4, 5 through 6.
Table 2.
Efficacy—improvement in the appearance of cellulite depressions
| Scoring by three‐blinded reviewers | Participants (N = 56) |
|---|---|
| Overall efficacy | |
| Baseline CSS score (Mean ± SD) | 3.41 ± 0.89 |
| 95% CI (Lower–upper) | (3.17–3.65) |
| Mean CSS reduction (Mean ± SD) | 1.01 ± 0.5 |
| 95% CI (Lower–upper) | (0.80–1.07) |
| p‐value (paired t‐test baseline vs. Wk‐12, two‐sided) | p < 0.0001 |
| Correctly identified photos | |
| % Correct ID of 12‐wk post photo—(2 of 3 Reviewers) | 96.4% |
| χ 2 (Expected = 50/50 chance) | <0.0001 |
| GAIS‐cellulite | |
| Improved, much improved, very much improved—(mean) | 90.9% |
| Unchanged—(mean) | 9.1% |
| Worse—(mean) | 0.0% |
Figure 3.
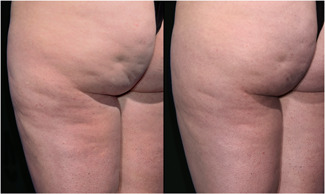
Images of Participant 63 showing (left to right) baseline cellulite severity (CSS = 3.33) and improvement after 12 weeks (CSS = 2.33). (Baseline weight: 143.4 pounds, Week‐12 weight: 144.0 pounds). CSS, Cellulite Severity Scale
Figure 4.
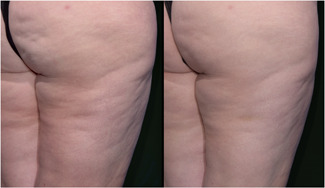
Images of Participant 57 showing (left to right) baseline cellulite severity (CSS = 5.00) and improvement after 12 weeks (CSS = 4.00). (Baseline weight: 186.0 pounds, Week‐12 weight: 192.4 pounds). CSS, Cellulite Severity Scale
Figure 5.
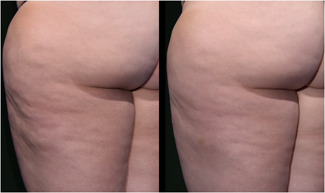
Images of Participant 58 showing (left to right) baseline cellulite severity (CSS = 4.67) and improvement after 12 weeks (CSS = 3.67). (Baseline weight: 191.4 pounds, Week‐12 weight: 194.8 pounds). CSS, Cellulite Severity Scale
Figure 6.
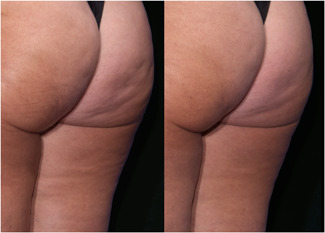
Images of Participant 26 showing (left to right) baseline cellulite severity (CSS = 4.33) and improvement after 12 weeks (CSS = 2.67). (Baseline weight: 138.0 pounds, Week‐12 weight: 140.0 pounds). CSS, Cellulite Severity Scale
3.3.2. Improvement in the appearance of cellulite skin laxity
The mean LS at baseline was 2.01 (on a 4 point scale). At week‐12 the mean LS was 1.44, a mean reduction of 0.57. This represented a 27.9% reduction from the baseline score. The difference between the baseline and 12‐week LS was statistically significant (p < 0.0001, t‐test, two‐tailed, paired). The Reviewers rated 67.3% of the treated sites as appearing improved, much improved or very much improved using a GAIS. Representative serial photographs demonstrating improvement in the appearance of skin laxity are shown in Figure 7.
Figure 7.
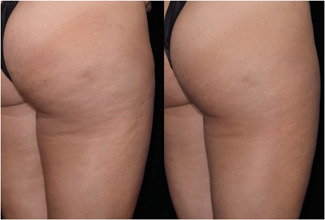
Images of Participant 18 showing (left to right) baseline laxity score (LS = 1.33) and improvement after 12 weeks (LS = 0.33). (Baseline weight: 139.0 pounds, Week‐12 weight: 133.8 pounds). LS, Laxity Score
3.3.3. Tolerability and satisfaction
The acoustic subcision procedure was reported tolerable to 55 of 56 (98.2%) participants (Table 4), and no participants requested that treatment be halted. On a 0–10 pain scale with 0 equal to no pain, the average pain for 1‐min treatment doses was 2.4 (range 0–6). The overall pain immediately after the treatment session was 0.3 (range 0–5). After viewing the baseline and 12‐week posttreatment photos of their own treated areas, 92.9% of participants agree or strongly agree that “the cellulite appears improved” and 76.8% of participants agree or strongly agree that “the treatment was relatively pain‐free” (Table 3).
Table 3.
Efficacy—Improvement in the appearance of cellulite skin laxity
| Scoring by three blinded reviewers | Participants (N = 56) |
|---|---|
| Overall efficacy | |
| Baseline laxity score (mean ± SD) | 2.01 ± 0.57 |
| 95% CI (Lower–upper) | (1.85 − 2.16) |
| Mean laxity reduction (mean ± SD) | 0.57 ± 0.40 |
| 95% CI (Lower–upper) | (0.46 − 0.67) |
| % Mean laxity reduction (Mean ± SD) | 27.9% ± 17.5% |
| p‐value (paired t‐test baseline vs. Wk‐12, two‐sided) | p < .0001 |
| GAIS—Laxity | |
| Improved, much improved, very much improved—(mean) | 67.3% |
| Unchanged—(mean) | 32.7% |
| Worse—(mean) | 0.0% |
3.4. Safety
The majority of participants, 53 of 56 (94.6%) had expected AEs including minimal or mild erythema (45, 80.4%); mild pain (5, 8.9%); mild folliculitis (2, 2.6%) and mild contusion (1, 1.8%). All AEs observed were expected, and all resolved without intervention. No participant experienced UAEs or SAEs.
4. DISCUSSION
A single 45–60 min acoustic subcision treatment resulted in 90.9% of the treated cellulite sites being graded as improved, much improved or very much improved using a GAIS, and Reviewers correctly identified the posttreatment photographs from 96.4% of treatments. There was also a 32.5% mean reduction in the 6‐point simplified CSS and a 27.9% mean reduction in the 4‐point LS.
Tissue release through disruption of fibrous septae can lead to meaningful improvement in the appearance of cellulite. Current approaches to providing tissue release have utilized the mechanical action of a reciprocated razor‐thin (0.45 mm) microblade, 2 thermoacoustic subcision of the septae with lasers, 7 or chemical subcision of the septae using collagenase enzyme injections. 8 These approaches are invasive, potentially painful, and/or associated with significant bruising. They potentially require local anesthesia during treatment, and bandages/compressive stockings posttreatment, which can result in extended down time.
Acoustic subcision treatment is noninvasive. The acoustic pulse's high‐frequency wavefront is designed to have a fast rise time (<100 ns) and mean peak output pressures up to 12 MPa. Fibrous septae are disrupted through acoustic shearing caused by the rapid acoustic pulses and not through painful cavitation or thermal tissue injury. The greater the pressure gradient across the septae (shearing), the more fibrotic disruption occurs. 9
Effective fibrotic disruption from acoustic shearing even with relatively high mean peak output pressures requires multiple acoustic pulses. Individual acoustic pulses do not produce sufficient shear to induce meaningful fibrous septae disruption. As discussed by Howard and Sturtevant, 10 membrane disruption is observed to increase progressively as the number of shock waves increases. Given that, the authors believe that effective acoustic subcision of fibrous septae requires 3000–12,000 acoustic pulses at each treatment site.
In addition to the number of acoustic pulses, the rate of applying those acoustic pulses is critical. Human skin is an anisotropic, nonlinear viscoelastic, loading history‐dependent material. 11 At a given acoustic pulse rate, the relaxation time for the tissue plays a role in the degradation from cumulative shock‐induced shearing. 12 At a pulse rate slower than the tissue relaxation time, there typically is no accumulated disruption. At a pulse rate faster than the tissue relaxation time, membrane disruption is observed, and this disruption increases progressively as the number of acoustic pulses increases. 10 Figure 8 provides a series of histological images of porcine subcutaneous tissue treated with acoustic subcision demonstrating increase disruption of fibrous septae with increased treatment doses.
Figure 8.
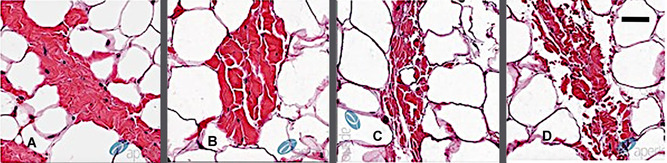
Fibrous septae disruption time‐dose response: Porcine adipose septae treated with an acoustic subcision device demonstrating increase disruption of fibrous septae with increasing 1‐min rapid acoustic pulse doses. (A) No treatment, (B) 1 min, (C) 2 min, and (D)3 min. Black bar = 50 microns
Equally important as the disruption of fibrous septae is the mechanical action of the rapid acoustic pulses on the dermal extracellular matrix that leads to a number of biological effects including neocollagenesis and angiogenesis. The direct and indirect stimulation of the dermal extracellular matrix and fibroblasts results in the remodeling of the dermis. 13 , 14 Figure 9 provides a series of histological images of porcine subcutaneous tissue treated with acoustic subcision demonstrating increased neocollagenesis. Inducing neocollagenesis will lead to improved appearance of skin laxity and cellulite.
Figure 9.
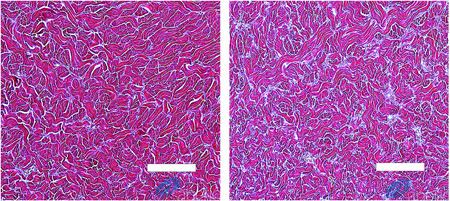
Dermal neocollagenesis: Herovici stained porcine reticular dermis from biopsies of sites treated with an acoustic subcision device. The 62‐days posttreatment histology image (right) demonstrates substantially greater amount of type II new collagen (light blue) in comparison to baseline histology image (left). White bar = 100 microns
Acoustic subcision disrupts fibrous septae and stimulates neocollagenesis through acoustic shearing and not cavitation or thermal injury. As a result, the potential for pain during treatment is minimized. In this study participants graded the pain during treatment as an average of 2.4 (on a 0–10 scale with 0 being no pain). Treatment discomfort, when it did occur, was typically over bony prominences and it was mitigated through repositioning of the participant and redirecting the treatment hand piece output. Following the acoustic subcision procedure, treatment discomfort, if any, did not persist. Immediately after the acoustic subcision procedure the participants graded the pain as an average of 0.3. Because there is minimal pain no anesthesia is required before, during or after the acoustic subcision treatment which saves total procedure time. Furthermore, since acoustic subcision is totally noninvasive the treatment can be undertaken by support staff within the dermatology office making it an efficient treatment procedure for both the office and the patient. Finally, no special posttreatment procedures or care needs to be provided. The patients can resume normal activities immediately following the procedure.
Limitations of this study include that only a single treatment session was provided to each participant, regardless of the severity of her cellulite. It is possible that multiple treatment sessions would lead to additional improvement, however, further clinical studies will be required to assess this. Every effort was made to recruit women of multiple ethnicities, however, 92.9% of the participants in this study were white. Additional studies should be undertaken to gain further evidence of efficacy in non‐White females.
5. CONCLUSION
A single noninvasive acoustic subcision session can safely provide meaningful improvement in the appearance of cellulite in terms of depressions, as well as skin laxity, with minimal treatment pain and no posttreatment down time. Further improvement in appearance is expected with multiple treatments over time. Additional trials to verify this are planned.
ACKNOWLEDGMENT
Funding and equipment for this study were provided by Soliton, Inc. (Houston, TX).
Tanzi EL, Capelli CC, Robertson DW, LaTowsky B, Jacob C, Ibrahim O, et al. Improvement in the appearance of cellulite and skin laxity resulting from a single treatment with acoustic subcision: Findings from a multicenter pivotal clinical trial. Lasers Surg Med. 2022;54:121–128. 10.1002/lsm.23448
REFERENCES
- 1. Christman MP, Belkin D, Geronemus RG, Brauer JA. An anatomical approach to evaluating and treating cellulite. J Drugs Dermatol. 2017;16(1):58–61. [PubMed] [Google Scholar]
- 2. Kaminer MS, Coleman WP, Weiss RA, Robinson DM, Coleman WP, Hornfeldt C. Multicenter pivotal study of vacuum‐assisted precise tissue release for the treatment of cellulite. Dermatol Surg. 2015;41(3):336–47. 10.1097/dss.0000000000000280 [DOI] [PubMed] [Google Scholar]
- 3. Casabona G, Pereira G. Microfocused ultrasound with visualization and calcium hydroxylapatite for improving skin laxity and cellulite appearance. Plastic and Reconstructive Surg. ‐ Global Open. 2017;5(7):e1388. 10.1097/gox.0000000000001388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaminer MS, Casabona G, Peeters W, Bartsch R, Butterwick K, Chao Y, et al. Validated assessment scales for skin laxity on the posterior thighs, buttocks, anterior thighs, and knees in female patients. Dermatol Surg. 2019;45:S12–21. [DOI] [PubMed] [Google Scholar]
- 5. Hexsel D, Fabi SG, Sattler G, Bartsch R, Butterwick K, Casabona G, et al. Validated assessment scales for cellulite dimples on the buttocks and thighs in female patients. Dermatol Surg. 2019;45:S2–11. [DOI] [PubMed] [Google Scholar]
- 6. Hexsel DM, Dal'forno T, Hexsel CL. A validated photonumeric cellulite severity scale. J Eur Acad Dermatol Venereol. 2008;23:523–8. [DOI] [PubMed] [Google Scholar]
- 7. DiBernardo B, Sasaki G, Katz BE, Hunstad JP, Petti C, Burns AJ. A multicenter study for a single, three‐step laser treatment for cellulite using a 1440‐nm Nd:YAG laser, a novel side‐firing fiber, and a temperature‐sensing cannula. Aesthet Surg J. 2013;33(4):576–84. 10.1177/1090820x13480858 [DOI] [PubMed] [Google Scholar]
- 8. Sadick NS, Goldman MP, Liu G, Shusterman NH, McLane MP, Hurley D, et al. Collagenase clostridium histolyticum for the treatment of edematous fibrosclerotic panniculopathy (cellulite): a randomized trial. Dermatol Surg. 2019;45:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lokhandwalla M, McAteer JA, Williams JC Jr, Sturtevant B. Mechanical haemolysis in shock wave lithotripsy (SWL): II. In vitro cell lysis due to shear. Phys Med Biol. 2001;46:1245–64. [DOI] [PubMed] [Google Scholar]
- 10. Howard D, Sturtevant B. In vitro study of the mechanical effects of shock‐wave lithotripsy. Ultrasound Med Biol. 1997;23(7):1017–22. [DOI] [PubMed] [Google Scholar]
- 11. Joodaki H, Panzer MV. Skin mechanical properties and modeling: A review. Proc Inst Mech Eng H: J Eng Med. 2018;232(4):323–43. 10.1177/0954411918759801 [DOI] [PubMed] [Google Scholar]
- 12. Freund JB, Colonius T, Evan AP. A cumulative shear mechanism for tissue damage initiation in shock‐wave lithotripsy. Ultrasound Med Biol. 2007;33(9):1495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22(1):73–80. 10.1016/s0945-053x(03)00004-0 [DOI] [PubMed] [Google Scholar]
- 14. Bass LS, Kaminer MS. Insights into the pathophysiology of cellulite: a review. Dermatol Surg. 2020;46:S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


