Figure 2.
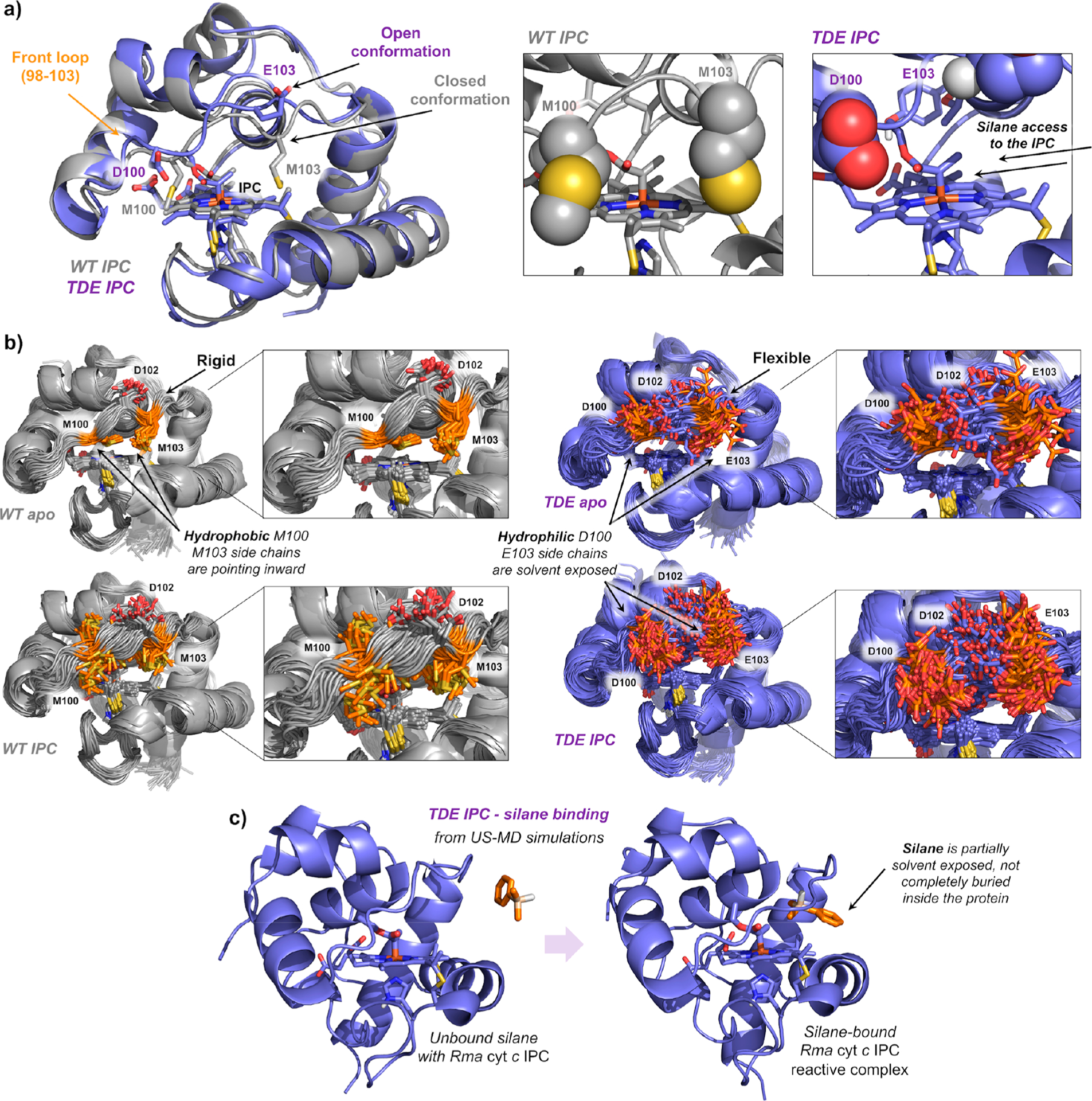
(a) Representative snapshots obtained from MD simulations of IPC bound in wild-type Rma cyt c (gray) and the Rma TDE variant (purple). Front loop M100/D100 and M103/E103 residues are represented in space filling format. Hydrophobic methionines (M100 and M103) close the substrate channel in wild-type Rma cyt c, but mutations M100D and M103E introduce hydrophilic residues that are exposed to the solvent in Rma TDE along the MD simulations, allowing an easier access of the silane substrate to interact with the IPC. V75T creates new key H-bond with Y71 that induces a more hydrophobic environment for the IPC intermediate. (b) Overlay of 50 snapshots (every 10 ns) obtained from 500 ns MD simulations for wild-type Rma cyt c and Rma TDE variants in its apo and IPC-bound states. Residues mutated in the front loop (original M100 and M103 in WT, and D100 and E103 in TDE variant) are shown in orange. Insets highlight the flexibility acquired by the front loop due to newly introduced mutations (going from “WT apo” to “TDE apo”) and the formation of the IPC (going from “WT apo” and “TDE apo” to “WT IPC” and “TDE IPC”, respectively). Corresponding RMSF analysis are reported in Figure S8. (c) Representative snapshots of silane binding to Rma TDE IPC in a catalytically relevant pose obtained from umbrella sampling MD simulations.
