Figure 3.
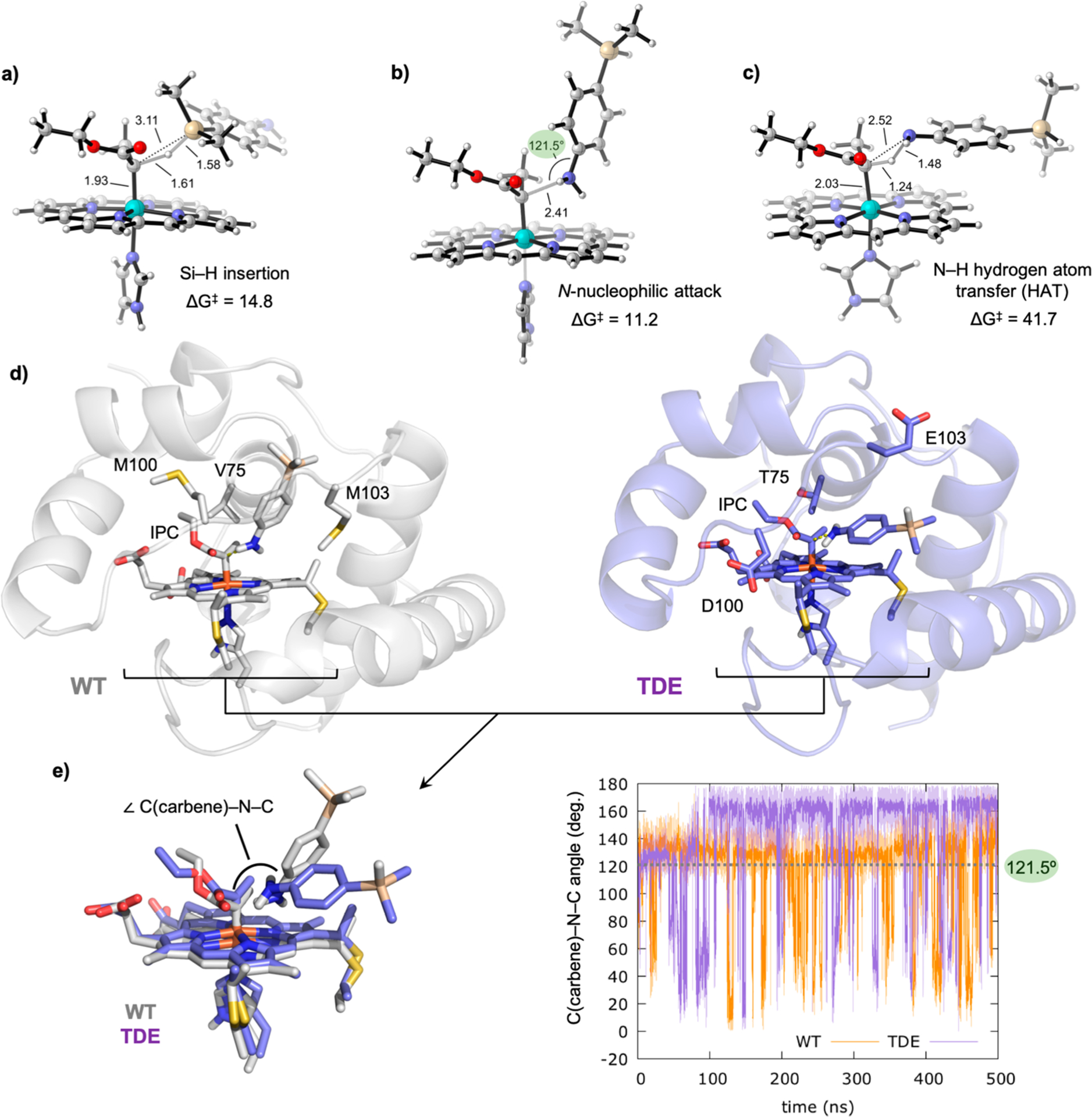
DFT optimized transition states using p-dimethylsilylaniline 6 as substrate: (a) Si–H carbene insertion; (b) N-nucleophilic attack to the electrophilic IPC carbon atom; (c) direct N–H carbene insertion. Gibbs free energies are estimated from lowest in energy OSS IPC 5 and are given in kcal·mol−1. Key distances and angles are given in Å and degrees, respectively. Different orientations of the substrate toward the carbene are required. (d) Analysis of the substrate binding pose and near attack conformations through constrained MD simulations (N(silane)–C(carbene) distance constrained along 500 ns MD trajectories). Representative snapshots of constrained MD simulations (constrained N–C distance to 2.5 Å, see Supporting Information methods section IIc for details) in the wild-type and TDE IPC Rma enzyme active sites are shown. (e) C(carbene)–N(silane)–C(silane) attack angles explored along the constrained MD simulations in wild-type (orange graph) and TDE (purple graph) IPCs. The ideal C(carbene)–N(silane)–C(silane) attack angle value for an effective N-nucleophilic attack is 121.5°, determined from DFT optimized TS.
