Summary
Background
Liver fibrosis accumulation is considered a turnover disease, with formation exceeding degradation, although this hypothesis has never been tested in humans.
Aims
To investigate extracellular matrix (ECM) remodelling in a biopsy‐controlled study of alcohol‐related liver disease (ALD) patients.
Methods
We evaluated the relationship between formation and degradation of four collagens as a function of histological fibrosis, inflammation and steatosis in 281 patients with ALD and 50 matched healthy controls. Post hoc, we tested the findings in a cohort of patients with alcohol‐related cirrhosis and assessed the collagens' prognostic accuracy. We assessed the fibrillar collagens type III (PRO‐C3/C3M) and V (PRO‐C5/C5M), the basement membrane collagen IV (PRO‐C4/C4M), and the microfilament interface collagen VI (PRO‐C6/C6M).
Results
Mean age was 54 ± 6 years, 74% male, fibrosis stage F0/1/2/3/4 = 33/98/84/18/48. Compared to controls, patients with ALD had higher levels of type III collagen formation and degradation, with the highest concentrations in those with cirrhosis (PRO‐C3 = 8.2 ± 1.7 ng/mL in controls, 14.6 ± 13.5 in ALD, 34.8 ± 23.1 in cirrhosis; C3M 7.4 ± 1.9 in controls, 9.3 ± 4.4 in ALD, 14.0 ± 5 in cirrhosis). ECM remodelling became increasingly imbalanced in higher stages of liver fibrosis, with formation progressively superseding degradation. This was particularly pronounced for type III collagen. We observed similar imbalance for inflammatory severity, but not steatosis.
Conclusions
ALD is characterised by both elevated collagen formation and degradation, which becomes increasingly imbalanced with more severe disease. Net increase in fibrillar collagens contributes to fibrosis progression. This has important implications for monitoring and very early identification of patients at highest risk of progressing to cirrhosis.
Alcohol‐related liver fibrosis is characterised by both elevated collagen formation and degradation, which becomes increasingly imbalanced with more severe disease.
![]()
1. INTRODUCTION
Globally, 2.4 billion people drink alcohol and more than 550 000 annual deaths from liver cirrhosis can be attributed to alcohol. 1 , 2 Alcohol‐related liver disease (ALD) is by far the most common cause of liver‐related mortality and morbidity, and yet less than 10% of excess drinkers progress to cirrhosis due to substantial inter‐individual variance in the rate and risk of progression. 3 , 4 , 5 This challenges identification of the patients in most need of intervention. For example, a recent study showed that 20% of ALD patients with simple steatosis on index biopsy, but no fibrosis, nevertheless died from liver‐related causes after an average of 10 years. 6 While today's non‐invasive fibrosis tests diagnose severe fibrosis with high accuracy, they are static and do not reflect the dynamics of pro‐ and anti‐fibrotic processes in the extracellular matrix (ECM), making them unsuited to assess disease progression rate. 7
We understand liver fibrosis accumulation as a pathological imbalance between the formation and degradation of ECM proteins. 8 A cirrhotic liver contains up to 10 times the amount of ECM proteins compared to a normal liver. 9 Hepatic ECM mainly consists of collagens, and the most abundant collagens of the liver are fibrillar collagens type I, III, V in the interstitial matrix, microfibrillar collagen type VI, and basement membrane collagens type IV and XVIII (Figure 1). 9 , 10
FIGURE 1.
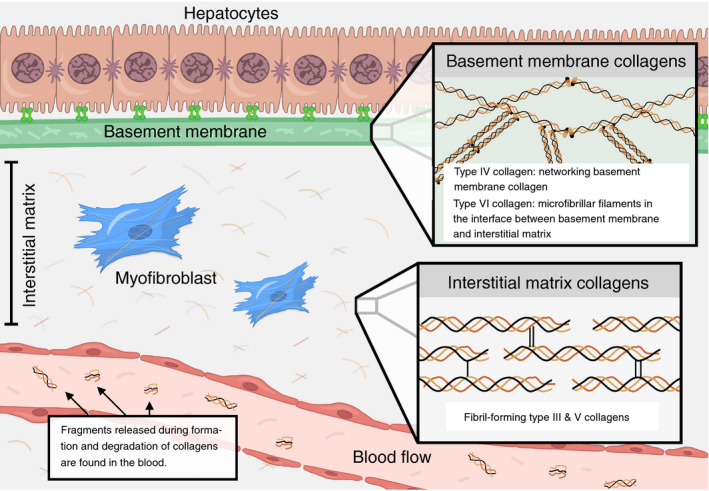
Collagens of the hepatic extracellular matrix (ECM) differ in function and localisation. Illustration depicting collagens type III, IV, V and VI, measured in this study. They are among the most abundant collagens of the liver and differ with regards to ECM function and localisation: Type III and V collagens are fibril‐forming collagens of the interstitial matrix, while the basement membrane's most important structural component is the networking type IV collagen. Type VI collagen is located at the interface between the basement membrane and the interstitial matrix and is made of microfibrillar filaments
During development of fibrosis, small neo‐epitope fragments that derive from the formation and degradation of ECM proteins are released to the circulation. Circulating markers of ECM turnover reflect spontaneous progression and regression of fibrosis in patients with hepatitis C or NAFLD, but there is a lack of studies in humans investigating the balance between collagen formation and collagen degradation, at the same time. 11 , 12 , 13 We hypothesised that the balance between neo‐epitope markers of ECM formation and degradation changes according to more progressive fibrosis stages in ALD.
We therefore aimed to investigate the formation and degradation of ECM collagens type III, IV, V and VI, as a function of fibrosis severity in a cohort of patients with asymptomatic ALD, compared to matched, healthy controls. Secondly, we aimed to correlate the collagen formation and degradation markers to hepatic inflammatory activity and steatosis score, and to investigate the effect of alcohol abstinence on ECM turnover.
Post hoc, we included a cohort of patients with compensated or decompensated cirrhosis with similar collagen measurements, and we assessed the ability of the collagen markers to predict all‐cause mortality.
2. PATIENTS AND METHODS
We performed a cross‐sectional, single‐centre study at Odense University Hospital in a cohort of consecutively recruited patients with biopsy‐verified ALD and a cohort of age‐, gender‐ and BMI‐matched healthy controls (ethics approval S‐20120071, S‐20160021, S‐20170087, S‐20160006G). We conducted the study in accordance with the principles of the 2013 Helsinki Declaration. All participants consented in writing at the time of inclusion, after oral and written information. The study is registered under OP_40 and OP_239 with Open Patient data Explorative Network (open.rsyd.dk).
Post hoc, we included a previously described cohort of patients with mostly decompensated cirrhosis referred to Hvidovre Hospital for liver vein catheterisation (ethics approval 2008‐41‐2020). 14 , 15
This study follows the STROBE checklist for cross‐sectional studies.
2.1. Patients
The target population was patients with ALD, described in detail elsewhere. 16 All were outpatients, either referred for investigations to our secondary care liver clinic, or recruited from primary care or the general population. From April 2013 to March 2017, we included patients with self‐reported ongoing or prior chronic alcohol use exceeding 14 units per week for women and 21 for men (12 g of alcohol per unit), for at least one year, 18‐75 years of age, and informed consent to a liver biopsy. We excluded patients with clear signs of cirrhosis (ascites, overt hepatic encephalopathy, large oesophageal varices with or without bleeding), severe alcoholic hepatitis, liver disease other than alcohol‐related, cancer, or comorbidity with an expected survival of less than a year.
We performed all investigations on the same day including physical examination, sampling of blood, medical history and questions on alcohol use. Patients' alcohol history and drinking pattern was assessed using the CAGE questionnaire 17 with additional questions to establish number of drinks in the week before inclusion, average weekly intake in the past 3 months and at the height of excessive use, and years of excessive alcohol intake. We defined abstinence as no alcoholic beverages for at least 1 week, and registered length of abstinence period in those who abstained from alcohol at the time of inclusion.
The cohort of patients with compensated or decompensated cirrhosis was included as part of a study investigating the correlation between collagen biomarkers and hepatic venous pressure gradient (HVPG). Patients had a diagnosis of cirrhosis based on either liver biopsy or clinical, biochemical, ultrasonic investigations and/or endoscopy. The majority were decompensated. We excluded patients with an aetiology other than alcohol‐related from our analyses.
2.2. Healthy controls
We matched a group of healthy controls with the ALD patients 6:1 on gender, age and BMI included between June 2016 and March 2017, recruited through online and paper advertisements in the municipality. Exclusion criteria were prior excessive use of alcohol, daily drinking, a weekly alcohol intake above low‐risk units (7/week for women, 14/week for men), binge drinking, any comorbidity including liver disease, substance abuse, any medication use other than occasional, mild pain relievers and use of antibiotics within the past 6 months. We also excluded participants if study investigations revealed any signs of chronic or acute disease. Finally, we conducted a chart review 6 months after inclusion and excluded participants who had experienced an event that indicated asymptomatic comorbidity at the time of inclusion (eg one had a myocardial infarction).
2.3. Quantification of biomarkers related to extracellular matrix remodelling
Neoepitope markers of collagen formation and degradation were evaluated in serum, with sample analysis blinded to clinical data. All biomarker assays were based on competitive ELISAs developed by Nordic Bioscience. The protocols were similar for all of the selected assays, and included coating 96‐well streptavidin plates with biotinylated synthetic peptides specific for the fragments of interest and incubating them for 30 minutes at 20℃. Next, we added 20 µL of standard peptide or pre‐diluted serum to designated wells, followed by peroxidase‐conjugated specific monoclonal antibodies and incubated them for 1 hour at 20℃ or 20 hours at 4℃ (depending on the assay). Then, tetramethylbenzidine was added to each well and the plates were incubated for 15 minutes at 20℃. The enzymatic reaction was stopped by the addition of 0.18 M H2SO4 and absorbance was measured at 450 nm with 650 nm as a reference. Calibration curves were calculated using a 4‐parametric fit model and the samples were measured within detection range and with acceptable coefficient of variation percentage (CV%).
2.4. Histology
We performed a percutaneous suction needle biopsy (17G Menghini needle: Hepafix, Braun) in all ALD‐patients, except the cirrhosis cohort. An experienced pathologist (SD) evaluated liver biopsies while blinded to other data. In the absence of cirrhosis, we used only biopsies with a length ≥10 mm and ≥5 portal tracts. We staged fibrosis according to Kleiner and scored steatosis (0‐3), lobular inflammation (0‐3) and ballooning (0‐2) according to the NAFLD activity score. 18 For a combined measure of hepatic inflammatory activity, we used the sum of lobular inflammation and ballooning (0‐5).
2.5. Follow‐up
We obtained mortality data through electronic health care records, with follow up of patients from inclusion until time of death or censoring on 1 October 2020, for the ALD cohort, or 6 May 2021, for the ALD cirrhosis post hoc cohort.
2.6. Statistical analyses
We report normal distributed data as means and standard deviations with between‐group comparisons using Student's t test; and non‐normal distributed data as medians and interquartile ranges with comparisons using Mann‐Whitney U test or Kruskal‐Wallis test. We used heatmaps to visualise how the eight markers of collagen formation and degradation correlated with histological lesions. We generated the heatmaps on the online R‐platform metaboanalyst.com after k‐Nearest Neighbour imputation of missing values (17 missing PRO‐C6 values), followed by log‐transformation and Pareto‐scaling to normalise the dataset. Next, we compared the differences between levels of collagen formation and degradation according to increasing fibrosis stage, inflammatory activity and steatosis score, one collagen type at a time, using the proportional change from healthy controls. We assessed whether the histological lesions were independently correlated with the four collagens in a multivariable linear regression model with the neoepitope collagen marker as dependent variable and fibrosis stage, hepatic inflammatory activity, and steatosis score as independent variables. To assess the prognostic ability of the collagen markers, we used Cox regression with Harrell's C‐statistics. We considered P‐values below 0.05 as statistically significant. Statistical analyses were calculated using STATA 16 (StataCorp).
3. RESULTS
3.1. Patients and controls
We included 281 ALD patients between May 2013 and March 2017, and 50 healthy controls from May 2016 to March 2017, matched on sex, age and BMI. The full cohort included 74% men, mean age 55 ± 10 years with a mean BMI of 26 ± 5 kg/m2. The ALD patients represented the full spectrum of fibrosis stages (Table 1). Approximately two‐thirds of patients reported excess drinking for more than 10 years (64%). A total of 144 (51%) ALD patients abstained from alcohol at the time of inclusion, the majority of whom had quit drinking within the year of study inclusion (77%, median 12 weeks abstaining). ALD patients exhibited higher levels of liver enzymes than controls, but the majority had values within the normal range, and liver function tests were comparable in the two groups. In line with this, a majority of ALD patients (213, 76%) had some degree of histological inflammatory activity evidenced by lobular inflammation and/or ballooning, but only 29% (82/281) presented with both ballooning and lobular inflammation, and a combined hepatic inflammatory activity score of at least three.
TABLE 1.
Patient characteristics
| Variables |
Alcohol‐related liver disease patients N = 281 a |
Healthy controls N = 50 |
P |
|---|---|---|---|
| Male gender | 208 (74%) | 37 (74%) | 1.000 |
| Age | 54 ± 6 | 56 ± 7 | 0.219 |
| BMI (kg/m2) | 26 ± 7 | 26 ± 4 | 0.709 |
| Type 2 diabetes | 40 (14%) | — | — |
| ALT (U/L) | 32 (21‐48) | 24 (21‐31) | <0.01 |
| AST (U/L) | 34 (25‐53) | 25 (22‐30) | <0.01 |
| Bilirubin (µmol/L) | 10 (7‐14) | 10 (8‐13) | 0.841 |
| GGT (U/L) | 78 (39‐216) | 24 (17‐29) | <0.01 |
| INR | 1.0 ± 0.13 | 1.0 ± 0.07 | 0.474 |
| Platelet count (109/L) | 241.1 ± 93 | 233.4 ± 39 | 0.568 |
| Abstaining from alcohol at time of inclusion | 144 (51%) | 2 (4%) | <0.01 |
| Less than 1 y of abstinence | 111 (40%) | — | — |
| Number of drinks in the week up to inclusion if not abstinent | 21 (7‐35) | — | — |
| Years of excessive drinking | 15 (8‐25) | — | — |
| Magnitude of alcohol consumption during overuse (drinks/week) | 105 (63‐175) | — | — |
| CAGE score (0‐4) b | 3 (2‐4) | — | — |
| Fibrosis stage 0/1/2/3/4 | 33/98/84/18/48 | — | — |
| Ballooning 0/1/2 | 145/84/52 | — | — |
| Lobular inflammation 0/1/2/3 | 74/122/65/20 | — | — |
| Steatosis 0/1/2/3 | 144/63/54/20 | — | — |
Characteristics of the 281 alcohol‐related liver disease patients and 50 healthy controls.
Summary data are reported as means ± SD, medians (IQR) or counts (%). P‐value reports comparisons between ALD patients and healthy controls using Student's test or Mann‐Whitney‐U‐test.
Abbreviations: ALD, alcohol‐related liver disease; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CAGE, Cut back, Annoyed, Guilty and Eye‐opener; GGT, gamma‐glutamyl transferase; INR, international normalised ratio.
The number of subjects is convenience‐chosen, selected to adequately reflect a representative cohort of ALD.
CAGE score is a screening questionnaire for alcohol use disorder, asking C: have you ever felt you needed to Cut‐down drinking? A: have people Annoyed you by criticising your drinking? G: Have you ever felt Guilty about drinking? E: Have you ever needed a drink first thing in the morning (Eye‐opener)?
The post hoc cohort of compensated or decompensated ALD cirrhosis included 83 patients: 75% men, age 57 ± 9 years, mean BMI of 25 ± 5 kg/m2, mean MELD score of 15 ± 6 and Child‐Pugh A/B/C of 29/30/24 (Table S1).
3.2. Differences in collagen formation and degradation between fibrosis stages
Patients with ALD had significantly higher levels of both collagen formation and collagen degradation compared to the matched healthy controls, for all collagen types except C5M (Table 2). Increasing fibrosis stage leads to a growing imbalance between collagen formation and degradation: While all eight collagen markers exhibited a positive correlation with fibrosis stage (Figure 2A), the formation of collagens type III, IV and V increased relatively more than degradation with higher fibrosis stages; the exception being collagen VI (Figure 3). This dysbalance in ECM remodelling was especially evident for the interstitial matrix type III collagen (Figure 3A). We observed a considerable, stepwise increase in the formation marker PRO‐C3 relative to controls, from 23% higher in F0 (IQR −1% to 52%; P = 0.003 for difference with controls) to 48% in F1 (16%‐83%; P = 0.023 for difference with F0), 99% in F2 (53%‐194%; P < 0.001), 208% in F3 (76%‐744%; P = 0.020) and 331% in F4 (194%‐486%; P = 0.205). In contrast, degradation marker C3M changed from 9% (IQR −13% to 43%) higher in F0 compared to controls, to 60% (IQR 46‐141) higher in F4, a substantially smaller change compared to PRO‐C3, and only statistically significant for F1‐F3 (P = 0.021 for F1 vs F2, P = 0.001 for F2 vs F3).
TABLE 2.
Biomarker characteristics
| Biomarker | Specification | Process | Cirrhosis | Alcohol‐related liver disease patients | Healthy | P |
|---|---|---|---|---|---|---|
| PRO‐C3 | Released N‐terminal pro‐peptide of type III collagen | Type III collagen formation | 34.9 (20‐48) | 14.6 (11‐24) | 8.2 (7‐9) | <0.001 |
| C3M | Neo‐epitope of MMP‐9 mediated degradation of type III collagen | Type III collagen degradation | 14.0 (11‐18) | 9.3 (8‐12) | 7.4 (6‐8) | <0.001 |
| PRO‐C4 | Internal epitope in the 7S domain of type IV collagen | Type IV collagen formation | 389.9 (320‐515) | 56.8 (35‐86) | 30.9 (24‐50) | <0.001 |
| C4M | Neo‐epitope of MMP‐2,9,12 mediated degradation of type IV collagen | Type IV collagen degradation | 154.6 (129‐213) | 31 (27‐39) | 27.4 (25‐34) | <0.001 |
| PRO‐C5 | Released C‐terminal pro‐peptide of type V collagen. | Type V collagen formation | — | 732.1 (585‐956) | 672.3 (542‐848) | 0.049 |
| C5M | Neo‐epitope of MMP‐2,9 mediated degradation of type V collagen | Type V collagen degradation | 168.3 (124‐242) | 6.3 (6‐7) | 6.2 (5‐8) | <0.001 |
| PRO‐C6 | C‐terminal of released C5‐domain of type VI collagen α3‐chain | Type VI collagen formation | — | 10.0 (8‐14) a | 7.3 (7‐8) | <0.001 |
| C6M | Neo‐epitope of MMP‐2 mediated degradation of type VI collagen | Type VI collagen degradation | 11.2 (9‐15) | 11.6 (9‐16) | 7.4 (6‐9) | <0.001 |
Characteristics of the collagen formation and degradation markers of extracellular matrix remodelling, and their levels in 281 patients with alcohol‐related liver disease vs 50 healthy controls matched for age, sex and BMI and 83 patients with alcohol‐related cirrhosis.
Serum concentrations (ng/mL) are reported as median and their interquartile range. P‐value reports comparisons between patients and healthy controls using the Mann‐Whitney U test for two‐group comparisons and Kruskal‐Wallis test for three‐group comparisons.
Abbreviation: MMP, matrix metalloproteinase.
PRO‐C6 data on 17 ALD patients are missing due to insufficient amount of serum for testing. PRO‐C5 and PRO‐C6 are not measured in the 83 cirrhosis patients.
FIGURE 2.
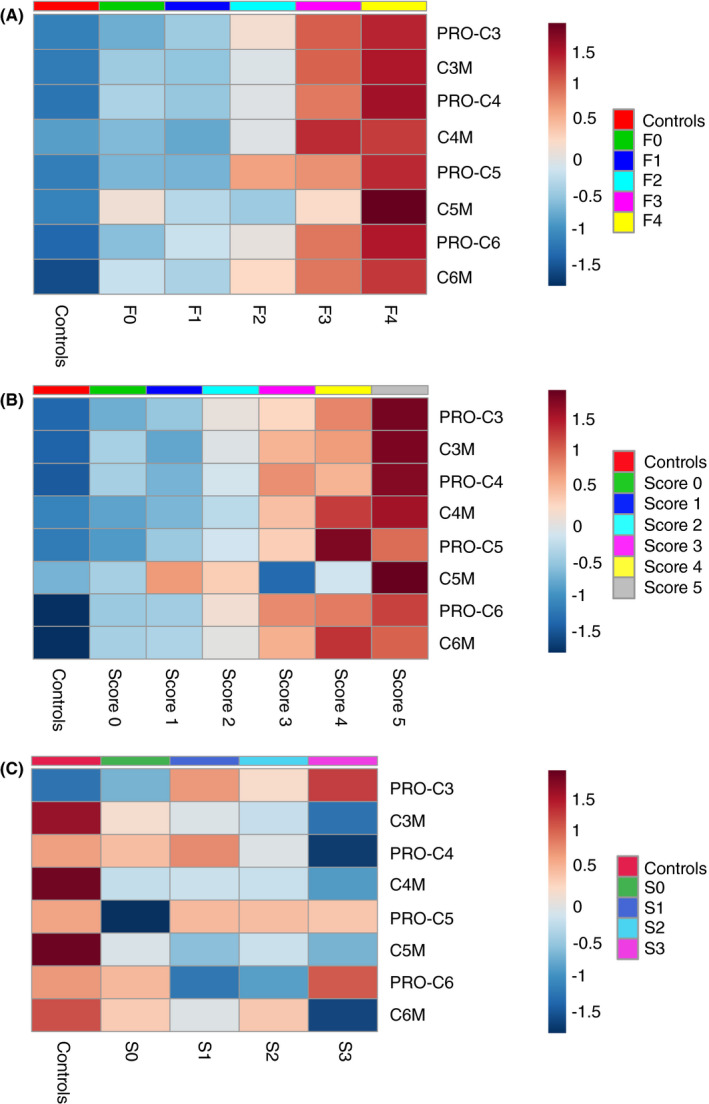
Heatmaps illustrating the concentration of the eight biomarkers according to (A) histological fibrosis stage, (B) hepatic inflammatory activity grade and (C) steatosis score with the 50 healthy, matched controls as comparator. The heatmaps visualise changes in normalised collagen concentrations along (A) fibrosis stages, (B) the sum of ballooning and lobular inflammation and (C) steatosis score. Blue illustrates that the average, normalised concentration in a group is below the overall average for all 281 alcohol‐related liver disease (ALD) and 50 controls, whereas red denotes a positive distance to the overall average. The figures show a positive correlation between all eight collagen markers and increasing fibrosis stages, and a similar positive correlation with hepatic inflammatory activity. The positive correlations are most evident for fibrosis stages F3‐4, and for the most severe inflammation sums 4‐5. Type V collagen degradation (C5M) exhibited a less clear pattern than the other collagen markers. For steatosis, type III collagen formation marker (PRO‐C3) was the only collagen marker that significantly increased in ALD patients when steatosis scores rose. The heatmaps are generated using the Metaboanalyst web‐based R packages with Pearson coefficients as distance measure and average (median) as clustering algorithm, after normalisation of data with log transformation and Pareto scaling
FIGURE 3.
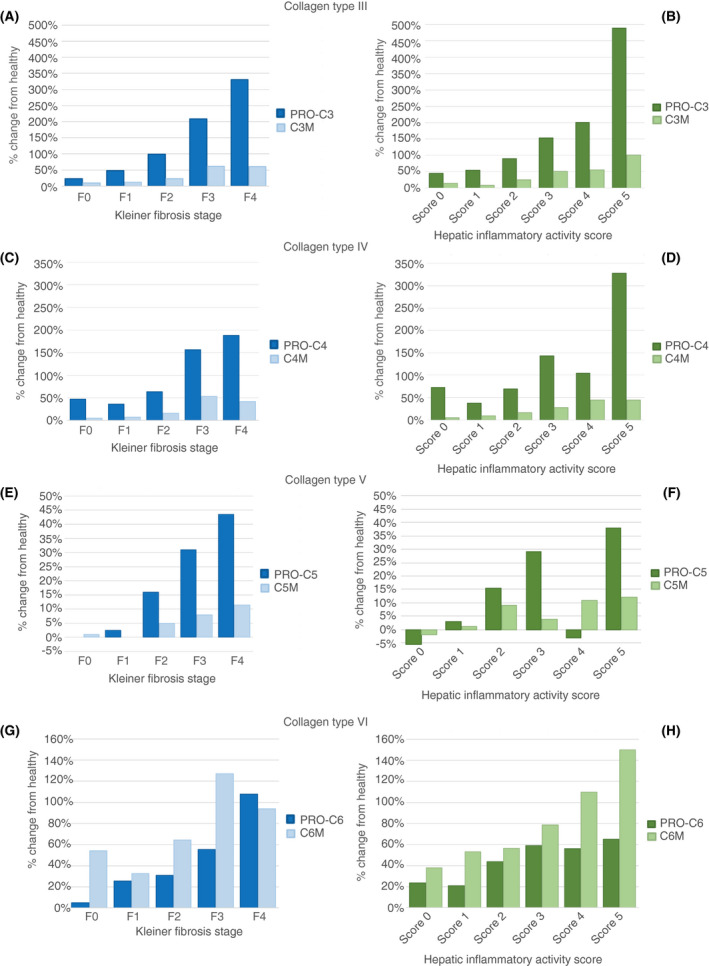
Relative changes in collagen turnover in 281 liver‐biopsied alcohol‐related liver disease (ALD) patients, using 50 healthy controls as reference. Proportional change for type III (A, B), type IV (C, D), type V (E, F) and type VI (G, H) collagen formation and degradation markers in patients with ALD, compared to the concentration in controls as reference. Stratified by Kleiner fibrosis stage (blue bars, left) or the hepatic inflammatory activity score (green bars, right). A positive percentage change indicates more formation and degradation of collagens in the ALD patients vs the healthy controls. We generated the hepatic inflammatory activity score by combining ballooning and lobular inflammation scores. C3M, C4M, C5M, C6M: collagen type III, IV, V and VI degradation markers; PRO‐C3, PRO‐C4, PRO‐C5, PRO‐C6: collagen type III, IV, V, and VI formation markers
The upregulation of collagen markers was most evident for fibrosis stages F3‐4 (Figure 2A), but a high proportion of patients with advanced fibrosis had concomitant moderate or severe hepatic inflammation (62% in F3‐4, vs 19% in F0‐2 patients). It was therefore not clear whether the positive correlation between collagen markers and fibrosis stage happened independently of hepatic inflammation. However, in multivariable analyses controlling for inflammatory activity and steatosis score, all markers except C5M correlated positively with fibrosis stage (P < 0.001). This independent relationship was also seen when comparing log‐transformed concentrations of the eight collagen markers within the ALD group, divided according to stage of fibrosis and grade of inflammatory activity (Figure 4).
FIGURE 4.
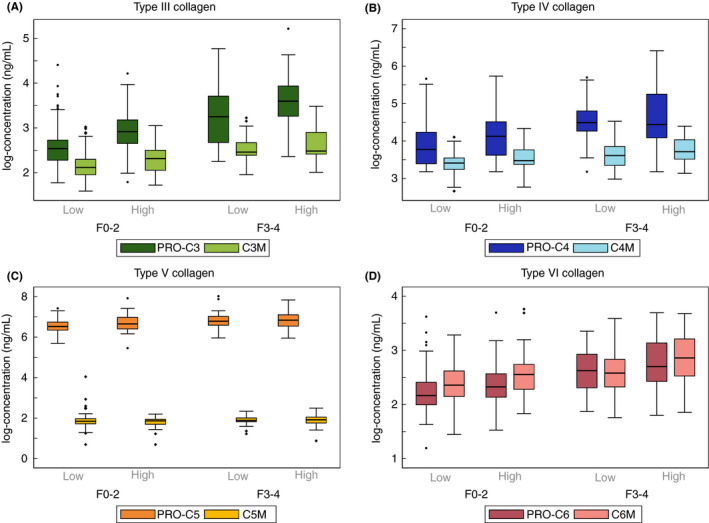
Changes in collagen formation and degradation according to both fibrosis and hepatic inflammation for collagens type III (A), type IV (B), type V (C) and type VI (D). Boxplots of collagen formation markers (PRO‐C3 to PRO‐C6) and collagen degradation markers (C3M to C6M), divided into fibrosis stages F0‐2 vs F3‐4, and subdivided according to hepatic inflammatory activity scores. Low denotes none or mild inflammation (score 0‐2). High denotes moderate or severe inflammation (score 3‐5). To compare formation and degradation markers, we log‐transformed their concentrations
3.3. Hepatic inflammatory activity and its correlation with collagen formation and degradation
Collagen formation and collagen degradation both increased with increasing severity of hepatic inflammation (Figure 2). As with fibrosis, we observed an increasingly dysbalanced ECM turnover, where collagen formation increasingly outweighed collagen degradation as inflammatory activity grew, except for collagen VI (Figure 3). Using type III collagen as example, PRO‐C3 relative to controls changed from a 45% increase in those with neither ballooning or lobular inflammation, to 489% increase in those with prominent ballooning and several foci of lobular inflammation. In contrast, C3M changed from 14% higher to 101% higher than healthy controls, for ALD patients without ballooning or lobular inflammation vs ALD patients with the most severe hepatic inflammatory activity.
Due to the interaction between fibrosis and inflammation, we also tested the correlation between collagen markers and inflammatory activity in multivariable analyses controlling for fibrosis stage and steatosis score: PRO‐C3, C3M, PRO‐C4 and C6M correlated positively with inflammatory activity, independent of fibrosis and steatosis (P ≤ 0.001). We detected similar trend when dividing inflammatory activity into mild vs moderate‐severe, and fibrosis into F0‐2 vs F3‐4 (Figure 4).
3.4. The effect of steatosis on collagen formation and degradation
PRO‐C3 was the only collagen marker that significantly increased in ALD patients when steatosis scores rose (Figure 2C). However, when controlling for fibrosis and inflammation in multivariable regression, this correlation disappeared.
3.5. The effect of abstinence and alcohol intake on collagen turnover
We did not detect any difference between ALD abstainers and ongoing drinkers in either of the collagen formation or degradation markers (median PRO‐C3 in abstainers = 14.4 ng/mL, IQR 11.0‐23.1, vs ongoing drinking = 15.0 ng/mL, 10.4‐25.6; C3M in abstainers = 9.6 ng/mL, 7.5‐12.3, vs 9.3 ng/mL, 7.5‐11.7). This remained the case in multivariable logistic regression controlling for fibrosis and inflammation, except for C3M, which showed a weak positive correlation with abstinence (odds ratio 1.06, 1.00‐1.13).
PRO‐C3 significantly correlated with the number of drinks in the week up to inclusion, independent of fibrosis stage and inflammation grade. Unadjusted, linear regression predicted that PRO‐C3 increased 0.33 ng/mL for every extra weekly unit of alcohol (correlation coefficient 0.33, 0.12‐0.55, P = 0.003). Drinking magnitude mainly affected PRO‐C3 due to high levels in 15 ALD patients (5% of the cohort) who reported an intake of 60 drinks or more in the week leading up to inclusion.
3.6. Collagen formation and degradation in patients with compensated and decompensated cirrhosis
In the cohort of patients with cirrhosis, we observed the same pattern of formation being relatively more increased than degradation for type III and IV collagens. The majority of the collagen markers had significantly higher levels than the 281 ALD‐patients with the exception of C6M. For PRO‐C4, C4M and C5M, we found an almost exponential upregulation with concentrations 7, 5 and 27 times higher in the cirrhosis cohort (Table 2).
3.7. The prognostic performance of collagen markers
During a median follow‐up time of 5.7 years (4.6‐6.7), 65 of the 281 patients with ALD (23%) died and eight patients were lost to follow‐up. PRO‐C3 had the highest prognostic accuracy with a C‐statistic of 0.728 (0.668‐0.789) (Table S2). The relative difference in upregulation between PRO‐C3 and C3M compared to controls, and C3M alone had lower prognostic performances (C‐statistic: 0.675 vs 0.670).
In the cohort of patients with compensated or decompensated cirrhosis, 74 patients (89%) died during a median follow‐up time of 4 years (1.5‐7.8). The collagen markers did not predict all‐cause mortality in this cohort neither did the relative difference in upregulation between formation and degradation markers (Table S3).
4. DISCUSSION
This study demonstrates that ALD leads to upregulation of both collagen formation and degradation, compared to healthy controls. In patients with compensated or decompensated cirrhosis, this upregulation was almost exponential. We thereby confirm that liver fibrosis is characterised by a highly dynamic damage‐repair process in the liver. Our findings indicate that fibrosis progression is not solely explained by increased collagen formation, but rather an increasingly dysbalanced ECM remodelling, where formation offsets degradation. Hepatic inflammation is presumably the main driver of collagen deposition, as ballooning and lobular inflammation independent of fibrosis stage correlated with higher levels of PRO‐C3, C3M, PRO‐C4 and C6M. In contrast, steatosis affected collagen turnover minimally, and steatosis is therefore more likely a sign of alcohol‐induced harm, rather than being harmful itself. When assessing prognostic performance, the collagen formation and degradation markers predicted all‐cause mortality in patients with asymptomatic disease but failed to do so in patients with advanced liver disease. Finally, alcohol and abstinence did not clearly affect the ECM dysbalance, except for higher levels of PRO‐C3 in the 5% heaviest drinking ALD patients, suggesting that other factors such as genetics and metabolic comorbidity may be important additional regulators of collagen turnover. In support of this hypothesis, we have previously shown that the majority of ALD patients exhibit several metabolic comorbidities, and that glucose intolerance is a stronger predictor of fibrosis severity than the magnitude of prior or ongoing drinking. 19 We also found elevated PRO‐C3 collagen formation in ALD patients without liver fibrosis on biopsy, compared to controls, suggesting a heightened damage‐repair process in the liver before histological evidence of harm.
Our findings provide novel understanding of the pathophysiology of liver fibrosis accumulation and regression in excess drinkers. Another important consequence of our findings regards future anti‐fibrotic interventions. Some patients with very low levels of tissue formation at higher fibrosis stages may be more amendable to an intervention stimulating fibrolysis, as compared to those with a clear fibrogenesis endotype. Pure anti‐fibrogenic drugs may be highly efficacious, as long as they do not impact collagen degradation. In support of this notion, we previously demonstrated that liver fibrosis patients with elevated levels of PRO‐C3 responded far better to an anti‐fibrogenesis drug compared to those with normal levels of PRO‐C3, albeit at a similar fibrosis stage. 11
This is the first study to combine paired markers of formation and degradation of four different types of collagens, representing different functions and localisations of the ECM. The fibrillar type III collagen exhibited the most prominent trends. Type III collagen is one of the most abundant collagens in the interstitial matrix of the diseased liver and has long been investigated as a biomarker for fibrotic liver diseases. 20 Our results confirm its importance, and support the use of collagen type III in the development of novel diagnostic algorithms. 21 In contrast, type V collagen exhibited markedly weaker associations. While type III and V are both fibril‐forming collagens of the interstitial matrix, there are structural, distributional and functional differences between the two, which may signify that they harbour distinct predictive abilities. 9 , 10 For instance, a study in hepatitis C patients found that fast progressors could be detected by high PRO‐C3 and C6M, and regressors by low PRO‐C5. 11
Our data show that formation of collagen type IV more than doubles in ALD patients with advanced fibrosis compared to healthy controls. Type IV collagen is a networking collagen in the basement membrane, offering support to the hepatocytes while allowing for the bidirectional flow of nutrients and metabolites from the blood. 9 The sinusoidal endothelium and biliary epithelium produces type IV collagen in the healthy liver. We speculate that portal fibroblasts and hepatic stellate cells may contribute to its production during liver injury. 22
Type VI collagen is made of microfibrillar filaments and is located between the fibrillar collagens at the interface between the basement membrane and the interstitial matrix. 9 Interestingly, the data from this study suggest that with increasing fibrosis stage, degradation of type VI collagen, C6M, increases more than its formation, PRO‐C6, signalling a net removal. PRO‐C6 not only provides structure, it is also a hormone known as endotrophin, which has been shown to promote cancer progression, fibrogenesis and metabolic syndrome—suggesting this as a potential common denominator of several fibrotic pathways. 9
Our findings are in line with a study in NAFLD patients using a different approach for assessing collagen remodelling than the neoepitope collagen markers used in this study. They observed a positive correlation between type I collagen remodelling and histological fibrosis stage, with an acceleration in collagen turnover with more advanced fibrosis. 23
Our results should be interpreted as exploratory, cross‐sectional findings that contribute to understanding progression mechanisms in alcohol‐related liver fibrosis.
Strengths include a large, single‐aetiology, biopsy‐controlled cohort with carefully selected age‐, gender‐ and BMI matched controls for comparison. The included ALD patients represented the full spectrum of disease, all were outpatients, asymptomatic and without significantly dysregulated biochemical liver function tests.
In conclusion, alcohol induces a significant upregulation of collagen formation as well as degradation. Severe alcohol‐related liver fibrosis is consequently not related solely to collagen deposition, but rather to an increasingly dysbalanced ECM remodelling, which favours collagen formation over degradation. This is particularly evident for the interstitial matrix collagen type III. Our findings may have implications for future prediction of disease progression, even at very early stages of fibrosis; and for the development of treatment strategies, which take differences in collagen turnover into consideration.
AUTHORSHIP
Guarantor of the article: AK.
Author contributions: MT, MK and AK conceptualised the study. SJ and MT drafted the manuscript. SJ and MT performed the data analyses. NSG, MJN, DJL, BSM, MKJ, SJA, SD, FB, SM and MT conducted the study. All authors revised the manuscript for important intellectual content and approved the final version of the article, including the authorship list.
Supporting information
Table S1‐S3
ACKNOWLEDGEMENTS
The authors thank the Odense Municipal Alcohol Rehabilitation Centre; the liver specialist nurses at Department of Gastroenterology and Hepatology; Open Patient data Explorative Network (OPEN); and our patient collaborators from the Danish Liver Patient Association and the Patient Advisory Board at Department of Gastroenterology and Hepatology.
Declaration of personal interests: NSG, MJN, DJL and MK are full time employees of Nordic Bioscience, Herlev, DK. MK and DJL own stock in Nordic Bioscience, Herlev, DK. MT, SJ, BM, MKA, SJA, FB, SM, SD and AK have nothing to disclose.
APPENDIX 1.
GALAXY Consortium
Manimozhian Arumugam, Peer Bork, Torben Hansen, Ema Anastasiadou, Christina Hartoft, Hans Israelsen, Morten Karsdal, Cristina Legido‐Quigley, Hans Olav Melberg, Maja Thiele, Jonel Trebicka, Aleksander Krag (coordinator).
Thiele M, Johansen S, Gudmann NS, et al; the GALAXY Consortium . Progressive alcohol‐related liver fibrosis is characterised by imbalanced collagen formation and degradation. Aliment Pharmacol Ther. 2021;54:1070–1080. 10.1111/apt.16567
The Handling Editor for this article was Professor Gideon Hirschfield, and it was accepted for publication after full peer‐review.
The members of GALAXY Consortium are listed in Appendix 1.
Funding information
This project has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement number 668031, Innovation Fund Denmark, Odense University Hospital's research funds, University of Southern Denmark's PhD funds and Region of Southern Denmark's postdoc funds. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Stine Johansen, Email: stine.johansen@rsyd.dk.
the GALAXY Consortium:
Manimozhian Arumugam, Peer Bork, Torben Hansen, Ema Anastasiadou, Christina Hartoft, Hans Israelsen, Cristina Legido‐Quigley, Hans Olav Melberg, and Jonel Trebicka
DATA AVAILABILITY STATEMENT
The full dataset is available by contact to open@rsyd.dk, after approval from the Danish Data Protection Agency.
REFERENCES
- 1. Griswold MG, Fullman N, Hawley C, et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151‐171. [DOI] [PubMed] [Google Scholar]
- 3. Kim D, Li AA, Gadiparthi C, et al. Changing trends in etiology‐based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology. 2018;155:1154‐1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pimpin L, Cortez‐Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718‐735. [DOI] [PubMed] [Google Scholar]
- 5. Parker R, Aithal GP, Becker U, et al. Natural history of histologically proven alcohol‐related liver disease: a systematic review. J Hepatol. 2019;71:586‐593. [DOI] [PubMed] [Google Scholar]
- 6. Hagström H, Thiele M, Roelstraete B, Söderling J, Ludvigsson JF. Mortality in biopsy‐proven alcohol‐related liver disease: a population‐based nationwide cohort study of 3453 patients. Gut. 2021;70:170‐179. [DOI] [PubMed] [Google Scholar]
- 7. Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and indirect markers in detection of advanced fibrosis in patients with alcoholic liver disease. Gastroenterology. 2018;154:1369‐1379. [DOI] [PubMed] [Google Scholar]
- 8. Schuppan D, Ashfaq‐Khan M, Yang AT, Kim YO. Liver fibrosis: direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68‐69:435‐451. [DOI] [PubMed] [Google Scholar]
- 9. Karsdal MA, Nielsen SH, Leeming DJ, et al. The good and the bad collagens of fibrosis ‐ their role in signaling and organ function. Adv Drug Deliv Rev. 2017;121:43‐56. [DOI] [PubMed] [Google Scholar]
- 10. Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531‐1546. [DOI] [PubMed] [Google Scholar]
- 11. Karsdal MA, Hjuler ST, Luo YI, et al. Assessment of liver fibrosis progression and regression by a serological collagen turnover profile. Am J Physiol Gastrointest Liver Physiol. 2019;316:G25‐G31. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma Pro‐C3 (N‐terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35:429‐437. [DOI] [PubMed] [Google Scholar]
- 13. Bril F, Leeming DJ, Karsdal MA, et al. Use of plasma fragments of propeptides of type III, V, and VI procollagen for the detection of liver fibrosis in type 2 diabetes. Diabetes Care. 2019;42:1348‐1351. [DOI] [PubMed] [Google Scholar]
- 14. Leeming DJ, Karsdal MA, Byrjalsen I, et al. Novel serological neo‐epitope markers of extracellular matrix proteins for the detection of portal hypertension. Aliment Pharmacol Ther. 2013;38:1086‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leeming DJ, Veidal SS, Karsdal MA, et al. Pro‐C5, a marker of true type V collagen formation and fibrillation, correlates with portal hypertension in patients with alcoholic cirrhosis. Scand J Gastroenterol. 2015;50:584‐592. [DOI] [PubMed] [Google Scholar]
- 16. Thiele M, Detlefsen S, Sevelsted Møller L, et al. Transient and 2‐dimensional shear‐wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology. 2016;150:123‐133. [DOI] [PubMed] [Google Scholar]
- 17. Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121‐1123. [DOI] [PubMed] [Google Scholar]
- 18. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 19. Israelsen M, Juel HB, Detlefsen S, et al. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol‐related liver fibrosis. Clin Gastroenterol Hepatol. 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20. Teare JP, Greenfield SM, Thompson R, et al. Comparison of serum procollagen III peptide concentrations and PGA index for assessment of hepatic fibrosis. Lancet. 1993;342:895‐898. [DOI] [PubMed] [Google Scholar]
- 21. Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: an algorithm incorporating PRO‐C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075‐1086. [DOI] [PubMed] [Google Scholar]
- 22. Karsdal MA, Detlefsen S, Daniels SJ, Nielsen MJ, Krag A, Schuppan D. Is the total amount as important as localization and type of collagen in liver fibrosis attributable to steatohepatitis? Hepatology. 2020;71:346‐351. [DOI] [PubMed] [Google Scholar]
- 23. Decaris ML, Li KW, Emson CL, et al. Identifying nonalcoholic fatty liver disease patients with active fibrosis by measuring extracellular matrix remodeling rates in tissue and blood. Hepatology. 2017;65:78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
The full dataset is available by contact to open@rsyd.dk, after approval from the Danish Data Protection Agency.


