Abstract
Background
Pain is an under‐recognized complaint among head and neck cancer (HNC) survivors. Treatment is hindered by inadequate characterization of pain.
Methods
A secondary analysis from a prospective, longitudinal study was conducted to characterize pain prevalence, quality, and functional consequences in 77 HNC patients. Pain and pain‐related outcomes were captured before treatment, at end‐of‐treatment, and 3, 6, 9 and 12 months post‐treatment.
Results
Pain was most prevalent at end‐of‐treatment and declined over time. Chronicity of pain was established by 6 months post‐treatment. Oral mucosal neuropathic pain was the most common chronic pain subtype at 12 months post‐treatment. Widespread joint and muscle pain was also present at lower numbers. 40.2% of patients continued to require analgesics at 12 months.
Conclusion
Peripheral and central pain subtypes contribute significantly to chronic pain in HNC survivors. Preventive and treatment regimens should be tailored to specific pain subtypes for optimal symptom control.
Keywords: chronic pain, head and neck cancer, mucositis, musculoskeletal, side effect
1. INTRODUCTION
Head and neck cancer (HNC) patients often undergo rigorous treatments, which involve chemotherapy, radiation, and surgery. Almost all HNC patients experience cancer or treatment‐related pain at some point along their treatment course. 1 Initially, pain in the HNC population manifests as acute, well‐localized nociceptive pain induced by the tumor or treatment. The etiology and mechanisms for acute pain have been well described and can usually be treated with Non‐Steroidal Anti‐Inflammatory Drugs (NSAIDs), acetaminophen, adjuvants such as gabapentinoids, and/or opioids. However, a subset of patients will continue to experience pain well beyond the course of treatment and transition to a chronic pain state. 2 Chronic pain, classically defined as pain that persists for more than 3 months after the expected period of healing, 3 has been poorly characterized in the head and neck survivor population. The etiology, mechanisms, and manifestations of chronic pain in the HNC population are complex, heterogeneous, and may evolve over time. The complexity and lack of data characterizing chronic pain in the HNC population hinders the development of effective treatment paradigms.
To address this gap, we undertook a secondary data analysis from a prospective, longitudinal descriptive study titled “Establishing Lymphedema and Fibrosis Measures in Oral Cancer Patients” (NIH/NIDCR 1R01DE024982). Study participants completed patient reported outcome measures that capture symptom burden including pain and pain‐related outcomes. The goal of this exploratory analysis was to characterize pain across the time course of treatment and recovery with an emphasis on pain subtypes for which longitudinal data is limited. A better understanding of chronic pain in the HNC population may help guide prevention and treatment, thereby improving outcomes.
2. MATERIALS AND METHODS
2.1. Trial design
The study (ClinicalTrials.gov identifier: NCT02412241) received approval by the Vanderbilt University Institutional Review Board and Vanderbilt‐Ingram Cancer Center Scientific Review Committee. Written informed consent was obtained from study participants prior to performing study‐related activities. The trial was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice.
2.2. Sample and settings
Patients were recruited at clinics that serve HNC patients within the Vanderbilt University Medical Center. Eligibility criteria included the following: (a) histologically proven oral cavity and oropharyngeal cancer; (b) Stage II or greater (using American Joint Committee on Cancer version 7); (c) treatment naïve; (d) ≥21 years of age; (e) able to speak and read English; and (f) willing and able to provide informed consent. Exclusion criteria included: (a) medical record documentation of cognitive impairment; (b) unwilling or unable to undergo schedule of follow‐up assessments; (c) presence of recurrent cancer; or (d) presence of an active second primary cancer.
2.3. Procedures
After signing the informed consent document, participants were assessed before treatment (baseline [BL]), end of treatment (EOT), and 3, 6, 9 and 12 months post‐treatment. EOT was defined as the first postoperative follow‐up visit for surgery only patients (approximately 2 weeks after surgery) and the first postradiation visit for all others. Postoperative assessments were delayed in a small number of patients for variable reasons. Demographic information was collected at BL. Disease and treatment‐related information were obtained after EOT through medical record review by study staff. Self‐report questionnaires were completed by the participants at the above noted assessment timepoints. Data were entered into the Research Electronic Data Capture (REDCap) database.
2.4. Measures
We describe only the measures reported in this analysis.
2.4.1. Demographic form
Demographic information includes patient characteristics such as age, gender, and ethnicity.
2.4.2. Medical information form
Disease and treatment‐related information includes primary site, cancer stage, histology, and treatment.
2.4.3. Short‐Form McGill Pain Questionnaire
This is a validated tool that includes two items that measure pain intensity and 15 words that provide descriptors of the sensory and affective qualities of pain. 4
2.4.4. Head and Neck Lymphedema and Fibrosis Symptom Inventory
This tool includes 33 items that encompass seven domains: swallowing and taste changes, soft tissue and neurologic toxicity, body image and sexuality, communication, mucosal irritation, systemic symptoms and social functioning, jaw and oral dysfunction. 5 Items include a stem answered as “yes” or “no.” If the participant endorses the symptom, they are then asked to rate the severity and bothersomeness on a scale of 1 (slight) to 5 (severe). This tool includes six pain‐related items.
2.4.5. Vanderbilt Head and Neck Symptom Survey Version 2.0 and General Symptom Survey
Vanderbilt Head and Neck Symptom Survey (VHNSS) Version 2.0 contains 50 items representing 10 subscales and three single items: nutrition, swallowing, speech/communication, pain, mucositis, mucosal sensitivity, xerostomia, nutrition, excessive mucus, taste change, dental health, smell, and range of motion. Cronbach's α for the 10 subscales range from 0.70 to 0.90 and has been validated against both patient reported and objective outcome measures. 6 , 7 The VHNSS v2.0 plus General Symptom Survey (GSS) includes 10 pain specific items that explore the causes and functional consequences of pain in the HNC population. The general pain subscale (GPS) of the VHNSS v2.0 plus GSS contains three items (average pain level over the last week, worst pain level over the last week, and pain causes difficulty sleeping). The mucositis subscale includes three items that address mouth and throat sores and their impact on swallowing and speaking. The mucosal sensitivity subscale that captures neuropathic pain and mucosal sensitivity and its impact. A single item addressed the efficacy of pain medications (opioids and nonopioid medications) if applicable. The GSS includes one item that assesses the systemic effects of disease and treatment. All items are scored on a scale of 0 (none) to 10 (severe).
2.5. Statistical analysis
Data were analyzed using IBM SPSS Statistics (version 26) and SAS (version 9.4). Patient demographic and treatment characteristics were summarized using descriptive statistics. Frequency distributions and graphical displays of the various types of pain assessments at each time of assessment were generated to describe prevalence and variability of those phenomena over the 12‐month post‐treatment study period. Where statistical tests were conducted, mixed‐effects generalized models were used to fit the most appropriate test distribution to the pain measure. Statistical significance was defined by p < 0.05.
3. RESULTS
3.1. Patient demographics and treatment characteristics
Demographic data and treatment characteristics of the patients (N = 77) are presented in Table 1. The median age was 59.3 years; most subjects were male (68.8%) and white (92.2%). The most common primary site was oropharynx (67.5%). Early and locally advanced disease were well represented (Stage II 35.1% and Stage IV 41.5%). Of the 72 patients for whom treatment information was available, 29.2% received surgery only and 69.4% received multimodality treatment. 66.6% received primary or adjuvant chemo‐radiation.
TABLE 1.
Sample characteristics (N = 77)
| Characteristics | Mean (SD) |
|---|---|
| Age (years) | 59.3 (10.1) |
| No. of patients (%) | |
| Gender | |
| Female | 24 (31.2) |
| Male | 53 (68.8) |
| Race | |
| White | 71 (92.2) |
| Black or African American | 4 (5.2) |
| Other | 2 (2.6) |
| Marital status | |
| Single/widowed/other | 18 (23.4) |
| Married/single, living with partner | 59 (76.6) |
| Highest education level | |
| Grade 12 | 28 (36.3) |
| Undergraduate | 37 (48.1) |
| Graduate | 12 (15.6) |
| Employment status | |
| Employed (by others) | 45 (58.4) |
| Self‐employed | 2 (2.6) |
| Not employed | 30 (39.0) |
| Area of residence | |
| City | 23 (29.9) |
| Country | 31 (40.3) |
| Suburb | 23 (29.8) |
| Primary tumor location | |
| Oral cavity | 25 (32.5) |
| Oropharynx | 52 (67.5) |
| Virus | |
| No known virus | 8 (10.4) |
| Human Papillomavirus | 46 (59.7) |
| Not tested | 23 (29.9) |
| Cancer stage | |
| I | 0 (0.0) |
| II | 27 (35.1) |
| III | 18 (23.4) |
| IV | 32 (41.5) |
| Complete cancer treatment received (N = 72) | |
| Surgery only | 21 (29.2) |
| Radiation only | 1 (1.4) |
| Surgery and radiation | 2 (2.8) |
| Chemo and radiation | 39 (54.1) |
| Surgery, chemo, and radiation | 9 (12.5) |
3.2. Prevalence and severity of pain over time
The distributions of the average and maximum score for the VHNSS v2.0 GPS are shown in Figure 1A. For comparison, the visual analog pain score from the Short‐Form McGill Pain Questionnaire (SF‐MPQ) is shown in Figure 1B. The median level of pain at BL was in the mild range as defined as pain score <4 (VHNSS GPS: median scores 1.0–2.0, Interquartile range (IQR) = 0–6; SF‐MPQ VAS: median = 9.0, IQR = 0–35). However, 31% of patients reported pain in the moderate to severe range (pain ≥4). Pain peaked at the EOT assessment period (VHNSS GPS median score 4.3, IQR = 1–7 [p < 0.001]; SF‐MPQ VAS median score 31.0, IQR = 7–63 [p = 0.018]). While the median pain scores decreased to BL levels (SF‐MPQ VAS median score 2.0, IQR = 1–15) or significantly below (VHNSS GPS median score 0.0, IQR = 0–2 [p < 0.01]) by 12 months post‐treatment, mild to moderate pain continued to be a persistent problem in a significant cohort of patients (40%). Thirty‐six percentage of patients reported widespread pain outside of the head and neck region at BL. This increased to 47% at 12 months post‐treatment but this change was not statistically significant (p = 0.637).
FIGURE 1.
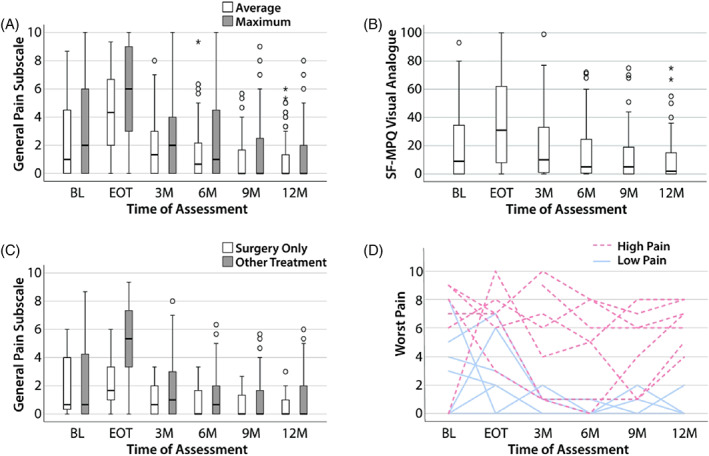
Pain is a common and significant complaint among HNC survivors. The median score for each timepoint is represented by horizontal bars. Circles represent 1.5 times the interquartile range; asterisks represent at least three times the interquartile range. (A) The general pain subscale is a composite of 13 pain‐related items from the VHNSS v2.0 plus GSS. The subscale is shown in average scores (white) and maximum scores (gray) at the timepoints indicated. (B) The SF‐MPQ visual analog scores were assessed at each of the timepoints indicated. (C) The general pain subscale is shown for surgery only patients (white) and multimodal therapy patients (gray). (D) Seven patients with worst pain scores of ≥4 at 12 months were selected (dashed red lines). Their worst pain scores were plotted over each timepoint. Seven additional patients with worst pain scores of <4 were also selected for comparison and their pain scores were plotted over time (solid blue lines). GSS, General Symptom Survey; HNC, head and neck cancer; SF‐MPQ, Short‐Form McGill Pain Questionnaire; VHNSS, Vanderbilt Head and Neck Symptom Survey [Color figure can be viewed at wileyonlinelibrary.com]
Median pain scores at BL were similar for patients treated with surgery only and multimodality therapy (both median = 0.67, IQR = 0–5, Figure 1C). There was a significant increase in median pain scores for those treated with multimodality therapy (p = 0.054). As shown in Figure 1C, the increase in median pain scores was most notable at EOT (surgery only: median = 1.7, IQR = 0–4; multimodality: median = 5.3, IQR = 3–8; p < 0.001, Figure 1C). Although the median pain scores were similar between the surgery only and multimodality treatment groups, the distribution of pain scores was wider in the multimodality treatment group.
To illustrate the pain trajectory of patients who experienced significant chronic pain, the worst pain scores of seven patients who exhibited moderate to severe pain (pain ≥4) at 12 months post‐treatment were plotted over time (Figure 1D, top). Most of these subjects continued to demonstrate moderate to severe pain at the 3‐, 6‐, and 9‐month follow‐up visits. Seven individuals with the lowest pain scores at 12 months follow‐up were also selected at random for direct comparison (Figure 1D, bottom). At 6 months, all subjects exhibited pain scores ≤2 and continued to have mild or no pain at 12 months.
3.3. Pain medication use over time
Pain medication use over time is shown in Figure 2A. Prior to receiving treatment, 46.8% (n = 36 of 77) reported taking pain medication. This increased to 79.2% by EOT and subsequently declined to 37.3% at 6 months post‐treatment. The percentage of patients reporting pain medication usage remained steady between 6 and 12 months post‐treatment (approximately 40%, Figure 2A). Of the patients who reported pain medication use, the average relief achieved from medications is summarized in Figure 2B. Prior to treatment, the median relief score was 5.0 (N = 36, IQR = 3–8). Median relief scores remain fairly stable through the EOT and 3 months post‐treatment when the largest proportion of the patients were using pain medication (EOT: N = 57, median relief = 6.0, IQR = 4–8; 3 months post‐treatment: N = 46, median relief = 5.5, IQR = 2–9). After 3 months post‐treatment, a smaller number of patients reported continued use of pain medications and pain relief varied widely within that group (N = 28–29, IQR for relief = 0–8) (Figure 2B).
FIGURE 2.
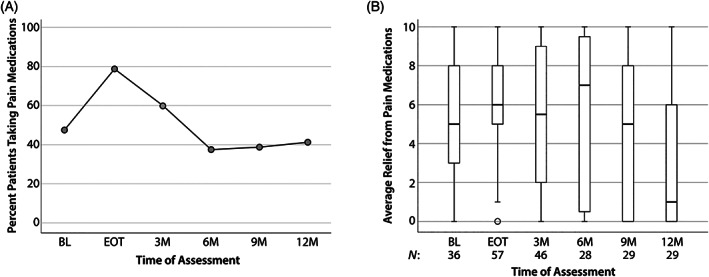
Pain medication use among HNC survivors. (A) Patients were asked about medications use for pain control (including nonopioids) at each timepoint shown. (B) The reported average relief from pain medications (for patients taking pain medications, shown as “N”) was assessed at each timepoint. Note that higher scores reflect better pain relief. The median score for each timepoint is represented by horizontal bars. Circles represent 1.5 times the interquartile range; asterisks represent at least three times the interquartile range. HNC, head and neck cancer
3.4. Prevalence of pain subtypes over time
Several items on the VHNSS v2.0 assess symptom burden related to mouth/throat sores (MTS), the results of which are shown in Figure 3A–C. While tumor and surgical wounds may be interpreted by patients as mouth or throat sores, these items largely reflect radiation‐induced mucositis. As shown in Table 1, 70% of the patients received radiation therapy as part of their treatment regimen. As expected, median MTS scores at EOT for the surgery only group were 0 (pain, IQR = 0–0), 0 (difficulty swallowing, IQR = 0–2), and 1 (difficulty speaking, IQR = 0–2). In contrast, the multimodality treatment group had median MTS scores of 6.0 (pain, IQR = 2–9), 6.0 (difficulty swallowing, IQR = 2–9), and 6 (difficulty speaking, IQR = 2–8). Mucositis‐related symptoms largely resolved by 3 months post‐treatment. Only a minority of patients exhibited pain and/or functional consequences from MTS beyond 3 months.
FIGURE 3.
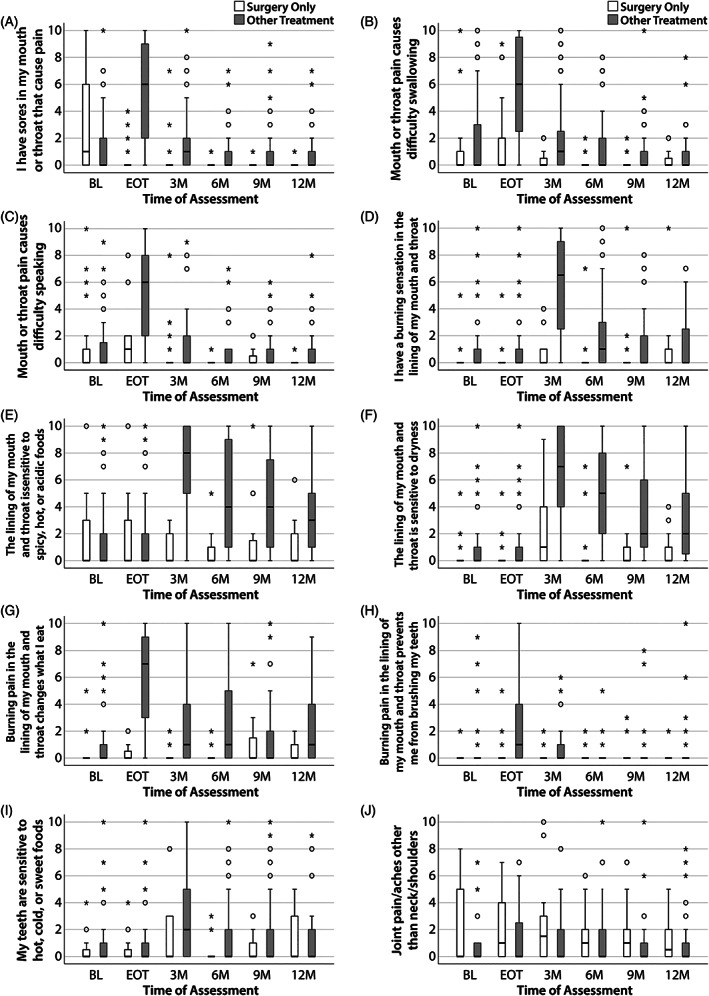
Pain‐related items from the VHNSS v2.0 plus GSS. Responses were analyzed in two groups: surgery only patients (white) and multimodality treatment patients (gray). The median score for each timepoint is represented by horizontal bars. Circles represent 1.5 times the interquartile range; asterisks represent at least three times the interquartile range. (A) I have sores in my mouth or throat that cause pain. (B) Mouth or throat pain causes difficulty swallowing. (C) Mouth or throat pain causes difficulty speaking. (D) I have a burning sensation in the lining of my mouth and throat. (E) The lining of my mouth and throat is sensitive to spicy, hot, or acidic foods. (F) The lining of my mouth and throat is sensitive to dryness. (G) Burning pain in the lining of my mouth and throat changes what I eat. (H) Burning pain in the lining of my mouth and throat prevents me from brushing my teeth. (I) My teeth are sensitive to hot, cold, or sweet foods. (J) Joint pain/aches other than neck/shoulders. GSS, General Symptom Survey; VHNSS, Vanderbilt Head and Neck Symptom Survey
Mucosal neuropathic pain (MNP) is manifested by burning pain and mucosal sensitivity. The mucosal sensitivity subscale of the VHNSS v2.0 includes five items that assess MNP and its functional consequences. The trajectory of individual items are shown in Figure 3D–H. Scores for all items were low at BL and EOT for all patients. Endorsement of mucosal burning increased dramatically in patients treated with multimodality therapy 3 months post‐treatment (median score >6, IQR = 2–9) (Figure 3D). The severity of mucosal burning subsequently declined through the 12‐month period. Sensitivity to certain types of food and dryness followed a similar but more pronounced pattern (Figure 3E,F). In addition, a significant cohort of patients had persistent mucosal sensitivity which was >4 in severity (6% to foods [4/61] and 20.2% to dryness [14 of 69]) at 12 months post‐treatment. The association between burning pain on dietary intake in the multimodality treatment group peaked at EOT and subsequently declined over the 12 month period but had minimal effect in the surgery only cohort (Figure 3G).
Few patients endorsed an adverse impact of mucosal sensitivity on dental hygiene at BL (11.8%) (Figure 3H). In the surgery only cohort, this number remained low across time. Conversely, this increased to 54.5% of subjects treated with multimodality therapy by the EOT (p < 0.05) with a subsequent decline to <10% in both groups at 12 months. Dental sensitivity to hot, cold, or sweet foods was uncommon and mild at BL or EOT (Figure 3I, median = 0, IQR = 0–1, 14.7% with scores >2 out of 10). Scores increased at 3 months post‐treatment with 39.7% of patients endorsing moderate to severe (>4 out of 10) dental sensitivity (statistically significant within the multimodality group, p < 0.05). By 12 months, 23% of all patients continued to report moderate to severe mucosal sensitivity.
Surgery only patients exhibited statistically significant higher reports of widespread pain than did patients who received multimodal therapy (main effect treatment, p = 0.006). As shown in Figure 3J, that effect was most apparent at BL and EOT. This declined by 3 months post‐treatment at which point only 24.5% of all patients (13/53) reported a score >2 out of 10. By 12 months, this further decreased to 16.3% (8/49).
3.5. Head and Neck Lymphedema and Fibrosis Symptom Inventory data
The Head and Neck Lymphedema and Fibrosis Symptom Inventory (HN‐LEF SI) assesses symptoms associated with soft tissue involvement by lymphedema and fibrosis. Discomfort, pain on movement, and tenderness (Figure 4A–C) increased between BL and EOT (p < 0.05) then subsided to BL levels by 3 months (p > 0.05) for both treatment groups. Discomfort remained prevalent at 12 months post‐treatment. Patterns of prevalence and severity of sore throat differed among treatment groups (p < 0.05, Figure 4D). Within the surgery only group, sore throat severity was relatively mild at BL and EOT. Only a few patients reported any degree of sore throat (≤15%) after EOT. Conversely, median sore throat severity scores were not only significantly higher within the multimodality treatment group when compared to surgery only patients at BL (median = 2 vs. median = 0), but they were also significantly increased to the moderate‐to‐severe range by EOT (median = 6, IQR = 3–9) (both p < 0.05) (Figure 4D).
FIGURE 4.
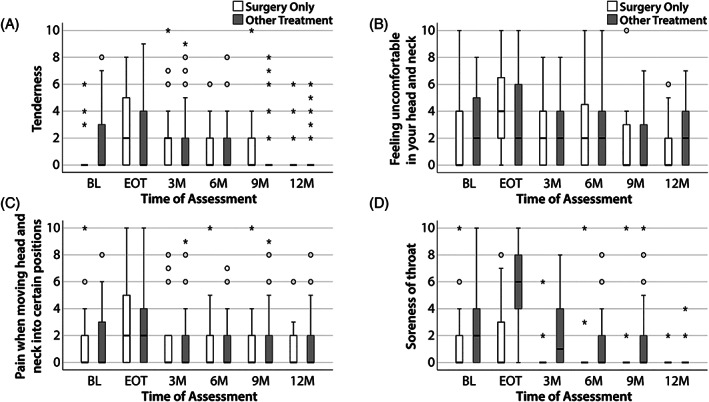
Pain‐related items from the HN‐LEF. Responses were analyzed in two groups: surgery only patients (white) and multimodality treatment patients (gray). The median score for each timepoint is represented by horizontal bars. Circles represent 1.5 times the interquartile range; asterisks represent at least three times the interquartile range. (A) Tenderness. (B) Feeling uncomfortable in your head and neck. (C) Pain when moving head and neck into certain positions. (D) Soreness of throat. HN‐LEF, Head and Neck Lymphedema and Fibrosis
4. DISCUSSION
In this longitudinal, hypothesis generating study, we describe pain in the HNC population at multiple timepoints throughout the treatment and post‐treatment period. Pain is ubiquitous in HNC patients, peaking at EOT in this study (Figure 1). Regardless of cause, pain will resolve or significantly improve for most patients by 3–6 months. Nonetheless, 40% of survivors experience chronic pain. For comparison, reports of chronic pain in the general population vary widely, ranging from 20% to as high as 53.8%. 8 , 9 Common etiologies of pain include joint pain/arthritis, low back pain, neck pain, and facial/jaw pain. Our results indicate that patients with pain at 3–6 months post‐treatment are likely to have transitioned to a chronic pain state. These numbers likely underrepresent the long‐term pain burden since 40% of patients continue analgesic use at 12 months (Figure 2). Patients receiving multimodality therapy are at highest risk for moderate to severe pain at all timepoints assessed when compared to the surgery only group. Finally, we provide descriptive data about pain subtypes unique to the HNC population using the VHNSS v2.0 plus GSS (Figure 3) and the HN‐LEF Symptom Inventory (Figure 4) tools. Nociceptive pain from mouth and throat sores (largely due to mucositis) was most prevalent at EOT and resolved by 3–6 months. The prevalence of mucosal sensitivity and burning pain peaked at 3 months and was associated with functional sequelae (sensitivity to foods, dryness) that persisted at 12 months. Widespread pain, a marker of central pain, was present in a small but significant percentage of survivors at 12 months (data not shown). Finally, patients frequently complained of the general feeling of discomfort and pain with movement of the head and neck (Figure 4).
Classically, the transition from acute to chronic pain has been defined as persistent or recurrent pain lasting beyond 3 months. This is an important distinction since patients with chronic pain often have components of central and/or peripheral sensitization or nonremediable pain generators that are a persistent source of nociceptive stimulus. In the HNC population, improvement in overall pain continues to be noted beyond 3 months. This may be due to the protracted recovery time from treatment‐related toxicities and the slow resolution of pain generators (Figures 3 and 4). By 6 months, however, the pain recovery curve flattens and the resolution of pain appears less likely, particularly for those with moderate to severe pain (Figure 1D). These patients are likely to have established chronic pain complaints by this time.
Pain medication use peaks at EOT, declines until 6 months, and then remains stable through 12 months (Figure 2). This supports the argument that chronic pain becomes established in this HNC population by the 6 months. The analgesic effects of these medications also tended to diminish over time with a particularly striking drop in efficacy at 12 months. This may be due to the preponderance of peripheral and central pain syndromes, which are particularly medication resistant. It may also be a result of the configuration of the tool itself since pain relief is the only item that is reverse scored. Further investigation is warranted to clarify this observation.
Mucositis pain arises from stimulation of peripheral nociceptors by inflammatory mediators released from damaged tissue. Once the mucosa heals, nociceptive pain resolves shortly thereafter. However, the presence of a protracted or exuberant inflammatory response may result in the development of peripheral sensitization. 10 In this setting, afferent signaling of these peripheral nerves continues even after the resolution of local nociceptive stimuli (Figure 3D–H). Alterations in central pain pathways at the level of the spinal cord may also contribute to persistent pain.
Mucosal burning and its functional consequences confer a significant burden to HNC survivors. While the prevalence of mucosal burning improves at 6 months (spontaneous pain), sensitivity of the mucosal lining to certain foods and dryness does not (provoked pain) (Figure 3E,F). In fact, these sensitivities are more impactful for patients than the pain itself at later timepoints. This suggests that patients may be experiencing allodynia (pain resulting from nonpainful stimuli), a marker of central sensitization and the commencement of a pathological process.
Previously, we also reported the results of a cross‐sectional study of 105 patients who were >12 months post‐treatment examining the late systemic symptoms experienced by HNC survivors. 11 Fifty‐three percentage of patients endorsed some degree of widespread pain outside of the head and neck region. This systemic symptom clustered with other systemic symptoms, such as fatigue, cognitive dysfunction, sleep disturbances, and temperature dysregulation. This constellation of symptoms is observed in central sensitivity syndromes such as fibromyalgia and chronic fatigue syndrome and may be a result of persistent neuroinflammation. 12 , 13 , 14 The release of peripheral pro‐inflammatory mediators by the cancer as well as the host response to both cancer and its treatment may contribute to neuroinflammation. We hypothesize that large tumors requiring aggressive therapy may be associated with more brisk and protracted inflammatory response and may therefore be more likely to result in central sensitivity syndrome. The prevalence of widespread pain was lower in this analysis compared to our previous report. This may be related to the prospective nature of the study, the inclusion of patients with less advanced disease, or our current practice of using gabapentin during radiation to ameliorate treatment‐associated symptoms. 15 Along with its neuromodulatory properties, gabapentin has been shown to have anti‐inflammatory effects in mice. 16 , 17 In fact, gabapentin has been recently proposed to have an antagonistic effect on N‐Methyl‐D‐aspartic acid (NMDA) receptors, which could theoretically halt the progression of central sensitization. 18 Further studies must be conducted to establish whether the phenomenon of widespread pain in HNC survivors falls within the category of central sensitivity syndromes.
Lymphedema and fibrosis (LEF), a common soft tissue complication of HNC therapy, is associated with a unique array of neurosensory symptoms including discomfort, tightness, stiffness, tingling, numbness, and tenderness. 19 , 20 These symptoms are classified as dysesthesias, unpleasant, or abnormal sensations, which may be spontaneous or provoked. Patients do not typically equate these symptoms with pain and thus they are often overlooked. Nonetheless, these symptoms may be bothersome or distressing to patients. Understanding the underlying pathophysiology of these symptoms may help yield effective preventive and treatment strategies.
There is a critical relationship between pain and function. Pain impacts oral intake which may result in dietary adaptations and poor diet quality. HNC patients who experience pain on movement of the jaw, neck, and shoulders will avoid inciting activities. Unfortunately, this may result in the adverse effects of disuse leading to a self‐perpetuating cycle of function loss. Similarly, odynophagia may prevent patients from using the muscles of deglutition resulting in long‐term swallowing abnormalities. Aggressive measures to prevent or control pain in HNC patients may not only decrease the incidence of chronic pain but may also prevent the development of long‐term functional deficits.
Although this secondary analysis has revealed several intriguing characteristics of acute and chronic pain in HNC survivors, the exploratory nature of this study is largely descriptive and hypothesis generating. Caution should be taken in interpreting this data in a definitive manner. In addition, other limitations include small sample size, single institutional study, predominantly male population, and sample size differences in treatment regimen subgroups. However, the prospective and longitudinal nature of this study provides valuable information at specific timepoints during survivorship. We employed multiple measures of pain and these measures were consistently gathered over specific timepoints, contributing to the uniqueness of the present study.
5. CONCLUSIONS
We were able to detect several distinct pain subtypes with differing etiologies in this longitudinal descriptive study. A high percentage of HNC survivors endorse persistent pain in addition to continued use of opioids and nonopioid analgesics for months after curative treatment. Further studies are warranted to longitudinally assess the impact of pain during the survivorship period.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number R01DE024982. Dianne I. Lou is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health under award number T32GM108554. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data sharing plan will adhere to NIH, NIDCR, and NIGMS policy guidance and standards set by the IRB at the study site.
Lou DI, Dietrich MS, Deng J, Murphy BA. Mechanisms of pain and their manifestations in head and neck cancer: Importance of classifying pain subtypes. Head & Neck. 2021;43(12):3720‐3729. doi: 10.1002/hed.26859
Section Editor: Katherine Hutcheson
Funding information National Institute of Dental and Craniofacial Research, Grant/Award Number: R01DE024982; National Institute of General Medical Sciences, Grant/Award Number: T32GM108554
DATA AVAILABILITY STATEMENT
The data sharing plan will adhere to National Institutes of Health and National Institute of Dental and Craniofacial Research policy guidance and standards set by the institutional review board at the study site. Data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Murphy BA, Wulff‐Burchfield E, Ghiam M, Bond SM, Deng J. Chronic systemic symptoms in head and neck cancer patients. J Natl Cancer Inst Monogr. 2019;2019(53):lgz004. [DOI] [PubMed] [Google Scholar]
- 2. Bouhassira D, Luporsi E, Krakowski I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain. 2017;158:1118‐1125. [DOI] [PubMed] [Google Scholar]
- 3. Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD‐11. Pain. 2015;156(6):1003‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Melzack R. The short‐form McGill Pain Questionnaire. Pain. 1987;30(2):191‐197. [DOI] [PubMed] [Google Scholar]
- 5. Deng J, Dietrich MS, Niermann KJ, et al. Refinement and validation of the head and neck lymphedema and fibrosis symptom inventory. Int J Radiat Oncol Biol Phys. 2021;109:747‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooperstein E, Gilbert J, Epstein JB, et al. Vanderbilt head and neck symptom survey version 2.0: Report of the development and initial testing of a subscale for assessment of oral health. Head & Neck. 2012;34(6):797‐804. [DOI] [PubMed] [Google Scholar]
- 7. Murphy BA, Dietrich MS, Wells N, et al. Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: A tool to assess symptom burden in patients treated with chemoradiation. Head & Neck. 2009;32(1):26‐37. [DOI] [PubMed] [Google Scholar]
- 8. Young RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. Forthcoming. 2021. 10.1097/j.pain.0000000000002291 [DOI] [PubMed] [Google Scholar]
- 9. Zajacova A, Grol‐Prokopczyk H, Zimmer Z. Pain trends among American adults, 2002–2018: patterns, disparities, and correlates. Demography. 2021;58:711‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism‐based approach to pain diagnosis. J Pain. 2016;17:T50‐T69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wulff‐Burchfield E, Dietrich MS, Ridner S, Murphy BA. Late systemic symptoms in head and neck cancer survivors. Support Care Cancer. 2019;27(8):2893‐2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339‐356. [DOI] [PubMed] [Google Scholar]
- 13. Lee BN, Dantzer R, Langley KE, et al. A cytokine‐based neuroimmunologic mechanism of cancer‐related symptoms. Neuroimmunomodulation. 2004;11:279‐292. [DOI] [PubMed] [Google Scholar]
- 14. Ridner SH, Dietrich MS, Sonis ST, Murphy B. Biomarkers associated with lymphedema and fibrosis in patients with cancer of the head and neck. Lymphat Res Biol. 2018;16:516‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith DK, Cmelak A, Niermann K, et al. Preventive use of gabapentin to decrease pain and systemic symptoms in patients with head and neck cancer undergoing chemoradiation. Head Neck. 2020;42(12):3497‐3505. [DOI] [PubMed] [Google Scholar]
- 16. Lee BS, Jun IG, Kim SH, Park JY. Intrathecal gabapentin increases interleukin‐10 expression and inhibits pro‐inflammatory cytokine in a rat model of neuropathic pain. J Korean Med Sci. 2013;28:308‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dias JM, de Brito TV, de Aguiar Magalhaes D, et al. Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation. 2014;37:1826‐1836. [DOI] [PubMed] [Google Scholar]
- 18. Chen J, Li L, Chen SR, et al. The α2δ‐1‐NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep. 2018;22:2307‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deng J, Murphy BA, Dietrich MS, Sinard RJ, Mannion K, Ridner SH. Differences of symptoms in head and neck cancer patients with and without lymphedema. Support Care Cancer. 2016;24:1305‐1316. [DOI] [PubMed] [Google Scholar]
- 20. Deng J, Ridner SH, Rothman R, et al. Perceived symptom experience in head and neck cancer patients with lymphedema. J Palliat Med. 2016;19(12):1267‐1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sharing plan will adhere to National Institutes of Health and National Institute of Dental and Craniofacial Research policy guidance and standards set by the institutional review board at the study site. Data that support the findings of this study are available on request from the corresponding author.


