Abstract
Background and Objectives
Surgery for colorectal cancer (CRC) negatively affects health‐related quality of life (HRQoL). Addressing shortcomings in literature, the purpose of this study was to evaluate the impact of surgery for CRC on the course of HRQoL from baseline up to 2 years after diagnosis.
Methods
In this prospective, population‐based study patients with newly diagnosed CRC were included between 2016 and 2019. HRQoL was assessed by the EORTC QLQ‐C30 questionnaire over time both between and within subgroups of patients that underwent right‐sided colonic, left‐sided colonic, and rectal resection using linear mixed model analyses.
Results
The study included 415 patients of whom 148 patients underwent right‐sided colonic (36%), 147 left‐sided colonic (35%), and 120 rectal resection (29%). Overall, HRQoL scores restored to baseline level 1 year after diagnosis. Impact of surgery seems to be more prominent in patients who underwent rectal resection, as they experienced more pain and had worse role and social functioning scores 4 weeks after surgery. Finally, among patients who underwent left‐sided and rectal resection, physical functioning did not return to baseline level during follow‐up.
Conclusion
This study shows several differences (between‐group and within‐group) in HRQoL according to surgery type and offers perspective which patients may need additional support in the care pathway.
Keywords: colorectal cancer, colorectal resection, health‐related quality of life, PROFILES, recovery, surgery
Abbreviations
- ANOVA
analysis of variance
- CRC
colorectal cancer
- DICA
Dutch Institute for Clinical Auditing
- DCRA
Dutch ColoRectal Audit
- EORTC QLQ‐C30
the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core module
- HRQoL
health‐related quality of life
- LMM
linear mixed model analyses
- NCR
Netherlands Cancer Registry
- PROFILES
Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship
1. INTRODUCTION
Colorectal cancer (CRC) is one of the four most common cancers in the world making it a major public health issue. 1 Health‐related quality of life (HRQoL) is negatively affected by CRC and it is being compromised further by the treatment modalities and associated adverse effects. 2 Incidence of CRC is increasing and patients tend to be diagnosed at a younger age. While new treatment options improve the overall survival rates, 2 an increasing amount of cancer survivors face persistent problems after primary treatment and live with this chronic disease. 3 As a result, quality of life has become an important outcome of surgery, the cornerstone for CRC treatment in current clinical practice. For some patients, a complicated, prolonged recovery due to treatment morbidity accompanied by a decline in HRQoL is not outweighed by the benefits of curation. 4
A number of studies have been published assessing HRQoL in patients with CRC. Some include assessment of quality of life only after treatment, 5 , 6 , 7 others include baseline assessment but have a relatively short follow‐up period of up to 6 months postoperatively. 8 , 9 , 10 Baseline assessment is necessary to interpret HRQoL results reported postoperatively and thus to determine the impact of treatment. Moreover, considering the fact that a substantial group of patients are likely to receive adjuvant treatment, a follow‐up period of 6 months will not represent the final HRQoL results. Only few studies have been published with both baseline and long term follow‐up results, 11 , 12 , 13 however, these studies included patients with rectal cancer only. To address these shortcomings, this study aimed to evaluate the course of HRQoL from baseline up to 2 years after diagnosis in patients with CRC undergoing surgery. Insight into the impact of CRC surgery on the course of HRQoL may help professionals to prepare and inform patients more optimally.
2. MATERIALS AND METHODS
2.1. Study design
The current study is a secondary analysis of the ongoing PROCORE study; a prospective, population‐based study in which patients with newly diagnosed CRC were included between 2016 and 2019. Longitudinal data is being collected on baseline (before start of treatment), 4 weeks postoperatively, and 1 and 2 years after diagnosis via PROFILES (Patient‐Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship), a registry containing data of the psychosocial and physical impact on cancer treatment. 3 The PROCORE study was ethically approved by the Medical Research Ethics Committees United (reference number NL51119.060.14). The current study is reported following the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
2.2. Population
Patients were recruited from four hospitals in the Netherlands: Catharina Hospital in Eindhoven, Elisabeth‐TweeSteden Hospital in Tilburg, Elkerliek Hospital in Helmond, and Máxima Medical Center in Veldhoven. Inclusion criteria consisted of adult patients with a pathologically confirmed diagnosis of CRC as a primary tumor. Patients were excluded in case of a previously diagnosed different cancer, except for basal cell carcinoma of the skin. Furthermore, patients unable to read or write the Dutch language or with cognitive impairments were excluded as well. Patients were eligible for the current analysis if they underwent surgical resection for CRC, completed the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire—Core module (EORTC QLQ‐C30) 14 at baseline and at least one follow‐up moment.
2.3. Data collection
CRC patients were informed and included before start of treatment by a case manager or research nurse. All patients provided written informed consent. Questionnaires could be filled out in paper‐and‐pencil versions or online via the PROFILES registry, according to patient preference.
PROFILES data were linked to data from the Netherlands Cancer Registry (NCR). Sociodemographic data (i.e., gender, age) and clinical information (e.g., tumor staging according to the American Joint Committee on Cancer 8th Edition, and neoadjuvant treatment) were obtained from the NCR. Questionnaires at baseline included questions regarding general characteristics (e.g., smoking status, educational level, and self‐administered comorbidity questionnaire 15 ). Information regarding the surgical treatment (e.g., type, surgical approach, stoma, and length of hospital stay) were collected from the Dutch ColoRectal Audit (DCRA), a national audit performed by the Dutch Institute of Clinical Auditing (DICA) in which information on all patients undergoing surgery for CRC is recorded. 16
For the current study, surgical procedures were divided into (1) right‐sided colonic resection (terminal ileum, cecum, ascending colon, hepatic flexure, and transverse colon), (2) left‐sided colonic resection (descending colon, splenic flexure, and sigmoid colon), and (3) rectal resection. Patients undergoing local excision, proctocolectomy, or subtotal colectomy were excluded due to the inability to divide into one of the subgroups.
2.4. Health‐related quality of life
HRQoL was assessed using the EORTC QLQ‐C30 (version 3.0). The EORTC QLQ‐C30 contains five functioning scales (physical, role, social, emotional, and cognitive functioning), a global quality of life scale, three symptom scales (fatigue, pain, and nausea/vomiting), and six single items (appetite loss, constipation, diarrhea, dyspnea, sleep disturbance, and financial impact). Participants scored items in an ordinal four or seven‐point Likert scale. The scores were converted into a scale ranging from 0 to 100 according to the EORTC scoring manual. 17 Higher scores on the functional scales and global QoL indicate better functioning and health status, while higher scores on the symptom scales indicate more symptoms.
For the current study, we included all functioning and global quality of life scales and selected the symptom scales and single items which were relevant for CRC patients in particular: fatigue, pain, sleep disturbance, constipation, and diarrhea.
2.5. Statistical analysis
Statistical analysis was performed using SPSS version 22 (SPSS Inc.). Baseline demographic and clinical characteristics were compared between patients that underwent right‐sided colonic resection, left‐sided colonic resection, and rectal resection. Categorical variables were compared between groups using χ 2 or Fisher's exact tests. Continuous variables were compared with one‐way analysis of variance (ANOVA) or Kruskal‐Wallis test, according to distribution of data. We evaluated changes in quality of life from baseline until 2 years after diagnosis by comparing scores of patients that underwent right‐sided colonic resection, left‐sided colonic resection, and rectal resection, both between and within groups. Linear mixed model (LMM) analyses were performed with the selected EORTC QLQ‐C30 scales as continuous dependent variables. We used a maximum likelihood estimation and an unstructured covariance matrix with a two‐level structure (i.e., patients and repeated time points). Time was analyzed as an independent categorical variable with four levels (i.e., baseline, 4‐week, 1‐year, and 2‐year follow‐up). Random intercepts on patient‐level were included in the models to take into account the intrasubject correlation between repeated measures. Analyses were adjusted for possible confounders which were selected as follows: baseline variables which were statistically different between groups or factors known to affect quality of life described in the literature. Values were derived from descriptive statistics, differences were based upon analyses adjusted for confounders. Clinical relevance of the changes of EORCT QLQ‐C30 scores was assessed for both between 18 and within groups 19 using previously published minimal important differences. A p = 0.05 or less was considered statistically significant.
3. RESULTS
Baseline questionnaires were completed by 477 of 713 (67%) patients eligible for participation in the PROCORE study. Of these, 14 patients had no surgical treatment and were therefore excluded for the current study. Seven patients were excluded from analyses due to surgery type (local excision, proctocolectomy, or subtotal colectomy). Finally, baseline EORTC QLQ‐C30 was not completed by six patients and 35 patients did not complete this questionnaire during at least one of the follow‐up moments resulting in 415 patients included for analysis (Figure 1).
Figure 1.
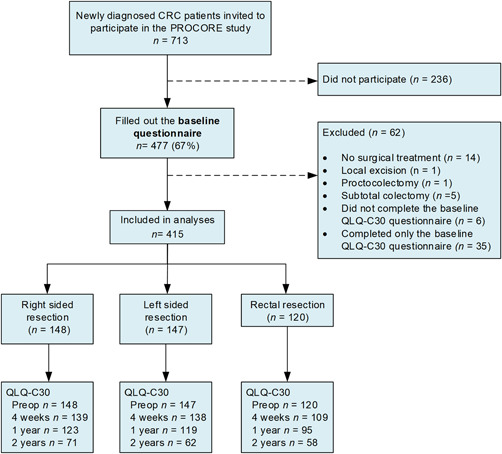
Flowchart of the study
3.1. Baseline characteristics
Table 1 shows the baseline demographic and clinical characteristics of 148 patients who underwent right‐sided colonic resection (36%), 147 patients who underwent left‐sided colonic resection (35%), and 120 who underwent rectal resection (29%). Significant differences were found between groups for age, time between diagnosis and completion of baseline questionnaire, number of comorbid conditions, tumor stage, (neo)adjuvant therapy, surgical approach, and stoma.
Table 1.
Baseline demographic and clinical characteristics of all patients that underwent colonic or rectal resection for colorectal cancer (n = 415)
| Variable | All respondents (n = 415) | Right‐sided resection (n = 148) | Left‐sided resection (n = 147) | Rectal resection (n = 120) | p value |
|---|---|---|---|---|---|
| Age at baseline (years) | 0.003 | ||||
| ≤70 | 248 (59.8%) | 76 (51.4%) | 86 (58.5%) | 86 (71.7%) | |
| >70 | 167 (40.2%) | 72 (48.6%) | 61 (41.5%) | 34 (28.3%) | |
| Gender (male) | 249 (60.0%) | 85 (57.4%) | 89 (60.5%) | 75 (62.5%) | 0.692 |
| Time between diagnosis and baseline (days) | 21 [15–28] | 21 [15–27] | 19 [13–26] | 24 [18–35] | 0.008 |
| Highest level of educationa | 0.344 | ||||
| Low | 41(9.9%) | 12 (8.1%) | 18 (12.2%) | 11 (9.2%) | |
| Medium | 265 (63.9%) | 89 (60.1%) | 99 (67.3%) | 77 (64.2%) | |
| High | 105 (25.3%) | 44 (29.7%) | 30 (20.4%) | 31 (25.8%) | |
| Missing | 4 (1%) | 3 (2.0%) | 0 (0%) | 1 (0.8%) | |
| BMI (kg/m2) | 0.362 | ||||
| <25 | 147 (35.4%) | 53 (35.8%) | 47 (32.0%) | 47 (39.2%) | |
| 25−30 | 161 (38.8%) | 57 (38.5%) | 55 (37.4%) | 49 (40.8%) | |
| ≥30 | 102 (24.6%) | 35 (23.6%) | 44 (29.9%) | 23 (19.2%) | |
| Missing | 5 (1.2%) | 3 (2.0%) | 1 (0.7%) | 1 (0.8%) | |
| ASA grade | 0.055 | ||||
| I–II | 354 (85.3%) | 119 (80.4%) | 131 (89.1%) | 104 (86.7%) | |
| III–V | 58 (14%) | 29 (19.6%) | 16 (10.9%) | 13 (10.8%) | |
| Missing | 3 (0.7%) | 0 (0%) | 0 (0%) | 3 (2.5%) | |
| Smoking | 0.542 | ||||
| Current smoker | 45 (10.8%) | 18 (12.2%) | 12 (8.2%) | 15 (12.5%) | |
| Former smoker | 241 (58.1%) | 83 (56.1%) | 84 (57.1%) | 74 (61.7%) | |
| Non‐smoker | 118 (28.4%) | 43 (29.1%) | 46 (31.3%) | 29 (24.2%) | |
| Missing | 11 (2.7%) | 4 (2.7%) | 5 (3.4%) | 2 (1.7%) | |
| Number of comorbid conditions | 0.012 | ||||
| 0 | 116 (28%) | 29 (19.6%) | 50 (34.0%) | 37 (30.8%) | |
| 1 | 135 (32.5%) | 46 (31.1%) | 46 (31.3%) | 43 (35.8%) | |
| ≥2 | 162 (39%) | 73 (49.3%) | 50 (34.0%) | 39 (32.5%) | |
| Missing | 2 (0.5%) | 0 (0%) | 1 (0.7%) | 1 (0.8%) | |
| Tumor stage (pTNM) | 0.004 | ||||
| I | 126 (30.4%) | 48 (32.4%) | 47 (32.0%) | 31 (25.8%) | |
| II | 115 (27.7%) | 51 (34.5%) | 42 (28.6%) | 22 (18.3%) | |
| III | 157 (37.8%) | 44 (29.7%) | 53 (36.1%) | 60 (50.0%) | |
| IV | 10 (2.4%) | 1 (0.7%) | 4 (2.7%) | 5 (4.2%) | |
| Unknown | 7 (1.7%) | 4 (2.7%) | 1 (0.7%) | 2 (1.7%) | |
| (Neo)adjuvant therapy (yes) | 150 (36.1%) | 35 (23.6%) | 48 (32.7%) | 67 (55.8%) | <0.001 |
| Radiotherapy only | 29 (7.0%) | 0 (0%) | 0 (0%) | 29 (24.2%) | |
| Chemotherapy only | 88 (21.2%) | 33 (22.3%) | 48 (32.7%) | 7 (5.8%) | |
| Chemo and radiotherapy | 33 (8.0%) | 2 (1.4%) | 0 (0%) | 31 (25.8%) | |
| Surgical approach | 0.002 | ||||
| Laparoscopy | 381 (91.8%) | 139 (93.9%) | 142 (96.6%) | 100 (83.3%) | |
| Open | 25 (6.0%) | 8 (5.4%) | 5 (3.4%) | 12 (10.0%) | |
| TaTME | 4 (1.0%) | 0 (0%) | 0 (0%) | 4 (3.3%) | |
| Missing | 5 (1.2%) | 1 (0.7%) | 0 (0%) | 4 (3.4%) | |
| Stoma (yes) | 73 (17.6%) | 1 (0.7%) | 3 (2.1%) | 69 (57.5%) | <0.001 |
| Diverting | 47 (11.3%) | 0 (0%) | 1 (0.7%) | 46 (38.3%) | |
| End | 25 (6.0%) | 1 (0.7%) | 2 (1.4%) | 22 (18.3%) | |
| Unknown | 1 (0.2%) | 0 (0%) | 0 (0%) | 1 (0.8% | |
| Length of hospital stay | 0.090 | ||||
| <10 days | 357 (86.0%) | 134 (90.5%) | 127 (86.4%) | 96 (80.0%) | |
| ≥10 days | 39 (9.4%) | 11 (7.4%) | 11 (7.5%) | 17 (14.2%) | |
| Missing | 19 (4.6%) | 3 (2.0%) | 9 (6.1%) | 7 (5.8%) |
Note: Values are in numbers (percentages) or median [IQR].
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range; kg, kilograms; m, meters; pTNM, pathological tumor node and metastasis stage; TaTME, transanal total mesorectal excision.
Level of education: low (no or primary school); medium (lower general secondary education or vocational training); high (pre‐university education, high vocational training, university).
3.2. Between‐group differences in HRQoL on different time points
At baseline, patients scheduled for right‐sided resection reported worse physical (p = 0.030) and role functioning (p = 0.008) compared with patients scheduled for left‐sided resection (Figure 2, 3 and Table SA). Furthermore, they were more fatigued than patients scheduled for left‐sided (p < 0.001) or rectal resection (p < 0.001), but reported less diarrhea (p = 0.007, p = 0.016, respectively). Finally, the scores on the insomnia scale were worse for patients scheduled for right‐sided resection compared with rectal resection (p = 0.035).
Figure 2.
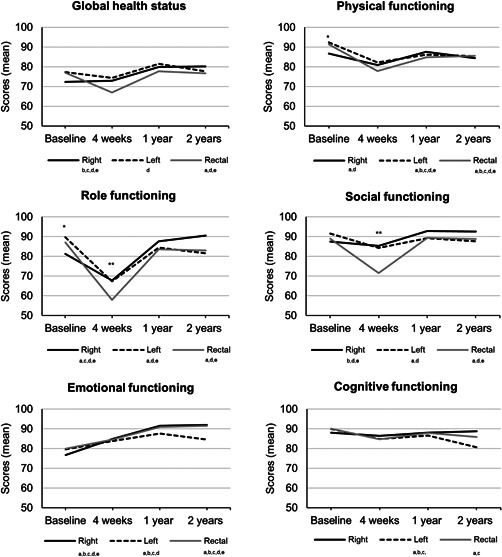
Health‐related quality of life function scores over time measured with the European Organization for Research and Treatment of Cancer QLQ‐C30 questionnaire for subgroups (right‐sided resection, left‐sided resection, and rectal resection). A higher score means better functioning/global health status. For a more visually suitable display of the results, the scale ranges from 50 to 100. The actual scale ranges from 0 to 100. Between‐group analyses: *significant difference between patients that underwent right‐sided and left‐sided resection. **significant difference between patients that underwent right‐sided and rectal resection. ***significant difference between patients that underwent left‐sided and rectal resection.Within‐group analyses: asignificant difference baseline, 4‐week follow‐up; bsignificant difference baseline, 1‐year follow‐up, csignificant difference baseline, 2‐year follow‐up; dsignificant difference 4‐week, 1‐year follow‐up; esignificant difference 4‐week, 2‐year follow‐up. Analyses were adjusted for: age, time between diagnosis and baseline, number of comorbid conditions, tumor stage, (neo)adjuvant therapy, surgical approach, and stoma
Figure 3.
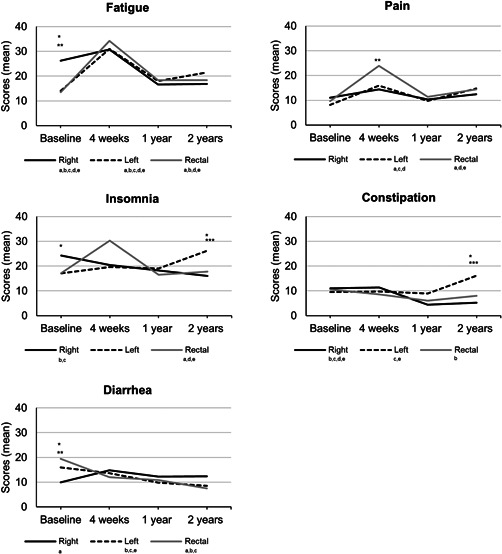
Health‐related quality of life of single items and scales over time measured with the European Organization for Research and Treatment of Cancer QLQ‐C30 questionnaire for subgroups (right‐sided resection, left‐sided resection, and rectal resection). A lower score means less symptoms. For a more visually suitable display of the results, the scale ranges from 0 to 40. The actual scale ranges from 0 to 100. Between‐group analyses: *significant difference between patients that underwent right‐sided and left‐sided resection. **significant difference between patients that underwent right‐sided and rectal resection. ***significant difference between patients that underwent left‐sided and rectal resection. Within‐group analyses: asignificant difference baseline, 4‐week follow‐up; bsignificant difference baseline, 1‐year follow‐up; csignificant difference baseline, 2‐year follow‐up; dsignificant difference 4‐week, 1‐year follow‐up; esignificant difference 4‐week, 2‐year follow‐up. Analyses were adjusted for: age, time between diagnosis and baseline, number of comorbid conditions, tumor stage, (neo)adjuvant therapy, surgical approach, and stoma
Four weeks postoperatively, patients who underwent rectal resection scored significantly worse on the role functioning scale compared with right‐sided resection (p = 0.039), on the social functioning scale compared with both groups (p = 0.001, p = 0.005), and had a higher level of pain compared with patients who underwent right‐sided resection (p = 0.049).
Scores did not significantly differ between groups 1 year after diagnosis.
Finally, after 2 years of follow‐up, patients who underwent left‐sided resection scored significantly worse compared with the other two groups on the insomnia (p = 0.031, p = 0.039) and constipation (p < 0.001, p = 0.016) scales.
All changes between groups except the change in physical functioning scale were clinically relevant.
3.3. Changes in HRQoL over time (within‐group differences)
3.3.1. Right‐sided colonic resection
For patients who underwent right‐sided resection, global health status, social functioning, and scores of the insomnia and constipation scales remained stable after 4 weeks compared to baseline but significantly improved after 1 and 2 years of follow‐up (Figure 2 and Table SA). A significant drop in scores at 4‐week follow‐up was observed in physical and role functioning, however, scores returned to baseline at 1‐year follow‐up. Emotional functioning was lowest at baseline but continued to improve from that point until the follow‐up after 2 years. Furthermore, more fatigue and diarrhea symptoms were reported 4 weeks after right‐sided resection, however, scores normalized to baseline levels or improved further at 1‐year follow‐up. Finally, cognitive functioning and pain scores did not change significantly over time. All changes were of clinical relevance.
3.3.2. Left‐sided colonic resection
Global health status remained stable compared to baseline 4 weeks after left‐sided resection, but significantly improved at 1‐year follow‐up. A deterioration in physical functioning, cognitive functioning, fatigue, and pain was seen 4 weeks postoperatively. These scores remained significantly lower than baseline score at 1 and 2‐year follow‐up. However, mean changes from baseline to 1 and 2 years of follow‐up for physical functioning were not clinically relevant. Furthermore, role functioning and social functioning scores deteriorated significantly after left‐sided resection, but returned to baseline level at 1‐year follow‐up. For emotional functioning, scores were lowest at baseline and continued to improve during follow‐up up to 1 year. A slight decrease in scores after 2 years of follow‐up was not clinically relevant. Scores for constipation initially remained stable after left‐sided resection but deteriorated at 2 years of follow‐up. Patients experienced less complaints of diarrhea over time in the follow‐up until 2 years. Finally, scores did not change significantly over time for insomnia.
3.4. Rectal resection
Global health status, role functioning, social functioning, pain, and insomnia initially deteriorated after rectal resection. However, scores improved and restored to baseline level after 1 year of follow‐up. For physical functioning, cognitive functioning, and fatigue symptoms the course was quite similar, however, scores did not recover to baseline level. Similar to the other two groups, emotional functioning was lowest at baseline but continued to improve during follow‐up after 4 weeks postoperatively and after 1 year. Patients undergoing rectal resection experienced less constipation‐related symptoms at 1 year of follow‐up compared with baseline. Finally, diarrhea‐related symptoms diminished over time after rectal resection. All reported changes were clinically relevant.
4. DISCUSSION
The current study evaluates HRQoL in patients with CRC and presents the impact of surgery on the course of HRQoL. In general, we found that HRQoL deteriorated at 4 weeks after surgery but restored approximately to baseline level at 1 year after diagnosis in most of the domains. Comparing results within patients that underwent right‐sided colonic, left‐sided colonic, and rectal resection over time, mean changes were largest in patients undergoing rectal resection. Notable results are discussed below.
HRQoL scores seemed to be most affected in patients undergoing rectal surgery. Recovery in HRQoL was less advanced 4 weeks postoperatively compared with the other subgroups suggesting that the amount of recovery is more extensive in this population. These findings are in agreement with those of Andersson and colleagues, who reported similar scores at 4 weeks after surgery 11 and are perhaps declared by dysfunctional bowel symptoms associated with rectal surgery, such as fecal incontinence, frequent bowel movements, emptying, and urgency difficulties. 7 The current study found the greatest differences in role and social functioning, and pain; scales that are closely related to bowel functioning according to previous studies. 7 , 20 In contrast to colonic procedures, tissue damage after rectal surgery is relatively high and patients often have a comprehensive perineal wound, which likely causes significant problems. Taken together, patients with rectal cancer should be informed about the more extensive recovery and additional support postoperatively may be indicated.
Around diagnosis, patients who underwent right‐sided resection reported more fatigue symptoms and worse physical functioning compared with the other two groups. This finding might be due to anemia often present in patients diagnosed with a tumor in the right‐sided colon. 21 Assessment of hemoglobin was not part of the PROCORE study protocol, therefore, this possible confounder could not be accounted for in the analyses. Patients should be screened routinely for anemia in the preoperative period and in case of insufficiencies be addressed to optimize hemoglobin levels and therewith possibly associated fatigue symptoms. 22
Considering physical functioning, for patients who underwent left‐sided or rectal resection, scores did not return to baseline levels after 2 years of follow‐up. A systematic review by Hamaker et al. showed similar results. 4 Physical functioning could be optimized using rehabilitation programs. Even better, the preoperative period may be more suitable to introduce interventions that improve a patient's functional capacity because the condition is generally better compared to the direct postoperative period. 23 A multimodal prehabilitation program (i.e., involving physical exercise, nutritional support including protein supplementation, lifestyle behavioral changes such as smoking and alcohol cessation, anemia correction, and mental support) diminishes the inevitable deterioration due to surgery and limits the extent. 24 Furthermore, because patients initiate the program in the preoperative period, continuation of such interventions are more easily resumed in the postoperative period. Prehabilitation enables patients to return more rapidly to baseline physical functioning.
An improved functional capacity both pre and postoperatively enables patients to maintain a certain level of independence; to continue to carry out daily activities in both personal and professional setting. Role functioning, the scale with most extreme changes over time in this study, reflects these daily activities and is, therefore, an important domain for the patient. Informing the patient about the impact of surgery on daily life activities is essential and furthermore, encourages initiatives such as prehabilitation.
Strengths of the current study are the large population‐based sample size and the prospective collection of data containing both preoperative and long‐term postoperative HRQoL values of a validated questionnaire. This study also has several limitations. First, we intended to include postoperative complications in our LMM analyses since this is expected to impact HRQoL to a great extent. However, since these data were not available, this was not possible. Instead, we considered to include length of hospital stay (< of ≥10 days) as a surrogate measure since a longer admission is generally caused by complications occurring in the direct postoperative period. Another confounder one could assume to be included in the analyses is American Society of Anesthesiologists (ASA)‐classification. Instead, we included number of comorbidities, which was significantly different between groups, as a confounder. We think that this is a better indicator of preoperative health status since comorbidities that might negatively impact HRQoL are included in this variable and do not contribute to a higher ASA‐classification. Second, conclusions from our HRQoL findings after 2 years of follow‐up should be made with caution due to the ongoing nature of the study. Based on the current data, we believe however that HRQoL does not change significantly after 1 year of follow‐up. Third, laparoscopy is widely implemented in the Netherlands and standard surgical approach in most surgeries (91% in the current study). However, since not all countries have similar rates of laparoscopic approach in CRC, generalization of the study results should be done with caution. Finally, although analyses were corrected for multiplicity within the various scales and items, we did not impose a corrected p‐value between those scales and items. Since we believe all scales and items can be interpreted separately, additional correction for multiplicity is unnecessary.
The results of this study enable specialists to inform patients about the impact of surgery on HRQoL domains in general and specified by surgery type. It helps patients to conceive realistic expectations. This crucial information should be communicated with the patient beside standard information regarding surgery procedure and risk of complications and is to date perhaps somewhat underexposed in the consultation room. Additionally, such results offer perspective which patients need additional support in what period of the cancer care pathway and enable professionals to act accordingly.
The current study found that patients generally recover to baseline level somewhere between 4 weeks postoperatively and 1 year after diagnosis. However, full recovery after for example 8 weeks or 8 months obviously affects the impact on the patient's life. Therefore, assessing quality of life more frequently in the direct postoperative period will provide insight in the recovery rate and may be addressed in future studies. Furthermore, nonmedical factors that affect quality of life (i.e., social determinants of life) should be included in future studies in this direction.
5. CONCLUSIONS
The results of the current study enable clinicians in daily clinical practice to inform CRC surgical patients on the course of specific HRQoL domains up to 2 years after diagnosis. Furthermore, this study offers recommendations for potential strategies such as anemia correction or (p)rehabilitation to optimize specific HRQoL domains. In addition to other clinical treatment outcomes (e.g., survival, postoperative complications), HRQoL directly affects the patient and should therefore be discussed thoroughly on a routine basis.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Cynthia S. Bonhof, Floortje Mols, Muriël Reudink, Gerrit D. Slooter, and Charlotte J. L. Molenaar made substantial contributions to conception and study design. Cynthia S. Bonhof, Floortje Mols, Loes Janssen, Muriël Reudink, and Charlotte J. L. Molenaar made substantial contributions to the acquisition and/or analysis of data. Charlotte J. L. Molenaar, Muriël Reudink, Floortje Mols, Gerrit D. Slooter interpreted the data. Muriël Reudink and Charlotte J. L. Molenaar primarily drafted the manuscript. Cynthia S. Bonhof, Floortje Mols, Loes Janssen, and Gerrit D. Slooter critically revised it for important intellectual content. All authors gave final approval for this version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SYNOPSIS
This study evaluates the course of health‐related quality of life in patients with colorectal cancer undergoing right‐sided colonic, left‐sided colonic, and rectal resection. The results offer perspective which patients may need additional support in the cancer care pathway.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We thank all patients, health care providers and research assistants for their participation in the study.
Reudink M, Molenaar CJL, Bonhof CS, Janssen L, Mols F, Slooter GD. Evaluating the longitudinal effect of colorectal surgery on health‐related quality of life in patients with colorectal cancer. J Surg Oncol. 2022;125:217‐226. 10.1002/jso.26685
Muriël Reudink and Charlotte J. L. Molenaar contributed equally to this study.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467‐1480. [DOI] [PubMed] [Google Scholar]
- 3. van de Poll‐Franse LV, Horevoorts N, van Eenbergen M, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188‐2194. [DOI] [PubMed] [Google Scholar]
- 4. Hamaker ME, Prins MC, Schiphorst AH, van Tuyl SA, Pronk A, van den Bos F. Long‐term changes in physical capacity after colorectal cancer treatment. J Geriatr Oncol. 2015;6(2):153‐164. [DOI] [PubMed] [Google Scholar]
- 5. Koëter T, Bonhof CS, Schoormans D, et al. Long‐term outcomes after surgery involving the pelvic floor in rectal cancer: physical activity, quality of life, and health status. J Gastrointest Surg. 2019;23(4):808‐817. [DOI] [PubMed] [Google Scholar]
- 6. Park J, Danielsen AK, Angenete E, et al. Quality of life in a randomized trial of early closure of temporary ileostomy after rectal resection for cancer (EASY trial). Br J Surg. 2018;105(3):244‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Heinsbergen M, Janssen‐Heijnen ML, Leijtens JW, Slooter GD, Konsten JL. Bowel dysfunction after sigmoid resection underestimated: multicentre study on quality of life after surgery for carcinoma of the rectum and sigmoid. Eur J Surg Oncol. 2018;44(8):1261‐1267. [DOI] [PubMed] [Google Scholar]
- 8. Koedam TW, van Ramshorst GH, Deijen CL, et al. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient‐reported quality of life and functional outcome. Tech Coloproctol. 2017;21(1):25‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCombie AM, Frizelle F, Bagshaw PF, et al. The ALCCaS trial: A randomized controlled trial comparing quality of life following laparoscopic versus open colectomy for colon cancer. Dis Colon Rectum. 2018;61(10):1156‐1162. [DOI] [PubMed] [Google Scholar]
- 10. van Zutphen M, Winkels RM, van Duijnhoven FJ, et al. An increase in physical activity after colorectal cancer surgery is associated with improved recovery of physical functioning: a prospective cohort study. BMC Cancer. 2017;17(1):74‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersson J, Angenete E, Gellerstedt M, et al. Health‐related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg. 2013;100(7):941‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keller DS, Reali C, Spinelli A, et al. Patient‐reported functional and quality‐of‐life outcomes after transanal total mesorectal excision. Br J Surg. 2019;106(4):364‐366. [DOI] [PubMed] [Google Scholar]
- 13. Couwenberg AM, Burbach JPM, Intven MPW, et al. Health‐related quality of life in rectal cancer patients undergoing neoadjuvant chemoradiation with delayed surgery versus short‐course radiotherapy with immediate surgery: a propensity score‐matched cohort study. Acta Oncol. 2019;58:1‐10. [DOI] [PubMed] [Google Scholar]
- 14. Aaronson NK, Ahmedzai S, Bergman B, et al. The european organization for research and treatment of cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365‐376. [DOI] [PubMed] [Google Scholar]
- 15. Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self‐administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 16. Van Leersum NJ, Snijders HS, Henneman D, et al. The dutch surgical colorectal audit. Eur J Surg Oncol. 2013;39(10):1063‐1070. [DOI] [PubMed] [Google Scholar]
- 17. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D & Bottomley A on behalf of the EORTC Quality of Life Group The EORTC QLQ‐C30 scoring manual (3rd ed.). Published by: European Organisation for Research and Treatment of Cancer; 2001.
- 18. Cocks K, King MT, Velikova G, Martyn St‐James M, Fayers PM, Brown JM. Evidence‐based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire core 30. J Clin Oncol. 2011;29(1):89‐96. [DOI] [PubMed] [Google Scholar]
- 19. Cocks K, King MT, Velikova G, et al. Evidence‐based guidelines for interpreting change scores for the European organisation for the research and treatment of cancer quality of life questionnaire core 30. Eur J Cancer. 2012;48(11):1713‐1721. [DOI] [PubMed] [Google Scholar]
- 20. Emmertsen KJ, Laurberg S. Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter‐preserving resection for rectal cancer. Br J Surg. 2013;100(10):1377‐1387. [DOI] [PubMed] [Google Scholar]
- 21. Holzner B, Kemmler G, Greil R, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13(6):965‐973. [DOI] [PubMed] [Google Scholar]
- 22. Munting KE, Klein AA. Optimisation of pre‐operative anaemia in patients before elective major surgery—why, who, when and how? Anaesthesia. 2019;74(Suppl 1):49‐57. [DOI] [PubMed] [Google Scholar]
- 23. Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937‐947. [DOI] [PubMed] [Google Scholar]
- 24. Scheede‐Bergdahl C, Minnella EM, Carli F. Multi‐modal prehabilitation: addressing the why, when, what, how, who and where next? Anaesthesia. 2019;74(suppl 1):20‐26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


