FIGURE 4.
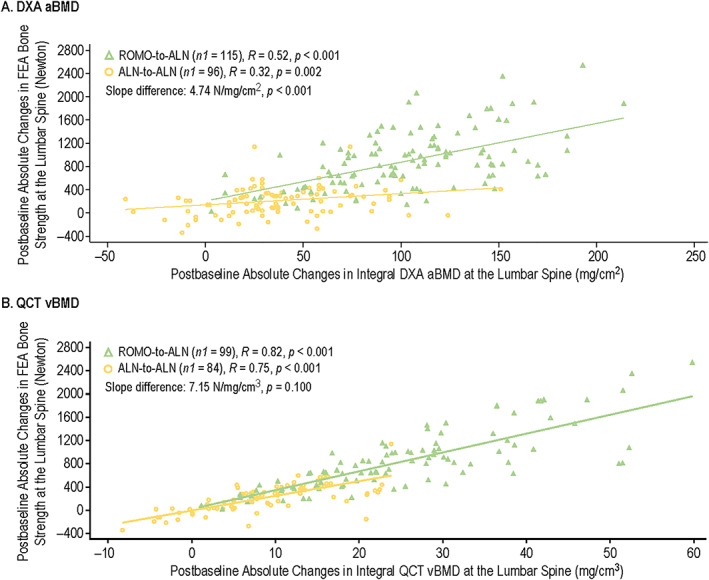
Correlation of postbaseline absolute changes in integral FEA bone strength and postbaseline absolute changes in integral DXA aBMD (A) and integral QCT vBMD (B) at the lumbar spine for romosozumab‐to‐alendronate and alendronate‐to‐alendronate groups through month 24. Includes randomized patients enrolled in the QCT/FEA imaging component of the ARCH substudy with baseline and one or more postbaseline reported results for the parameters of interest. n1 = number of evaluable measurements, with one or more measurements per patient. Month 6 and month 12 measurements were during the double‐blind period where patients received monthly romosozumab 210 mg sc or weekly oral alendronate 70 mg for 12 months; month 24 measurements were during the open‐label period when patients received open‐label weekly oral alendronate 70 mg for 12 months. Abbreviations: aBMD, areal bone mineral density; ALN, alendronate; ARCH, Active‐Controlled Fracture Study in Postmenopausal Women With Osteoporosis at High Risk; DXA, dual‐energy x‐ray absorptiometry; FEA, finite element analysis; QCT, quantitative computed tomography; R, Spearman's correlation coefficient; ROMO, romosozumab; vBMD, volumetric bone mineral density.
