FIGURE 3.
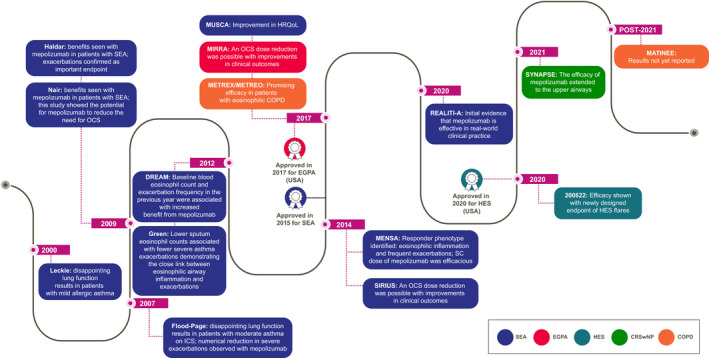
Timeline of the Phase III trials and approvals of mepolizumab in eosinophil‐driven diseases and the key takeaways. COPD, chronic obstructive pulmonary disease; CRSwNP, chronic rhinosinusitis with nasal polyposis; DREAM, Dose Ranging Efficacy And safety with Mepolizumab in severe asthma; EGPA, eosinophilic granulomatosis with polyangiitis; HES, hypereosinophilic syndrome; HRQoL, health‐related quality of life; ICS, inhaled corticosteroids; MATINEE, Mepolizumab as Add‐on Treatment IN participants with COPD characterized by frequent Exacerbations and Eosinophil level; MENSA, MEpolizumab as adjunctive therapy iN patients with Severe Asthma; METREO, MEpolizumab vs. placebo as add‐on TReatment for frequently exacerbating COPD patients characterized by EOsinophil level; METREX, MEpolizumab vs. placebo as add‐on TReatment for frequently EXacerbating COPD patients; MIRRA, Mepolizumab In Relapsing or Refractory EGPA; MUSCA, Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma; OCS, oral corticosteroids; REALITI‐A, REAL world effectiveness of mepolizumab In paTIent care—Asthma; SC, subcutaneous; SEA, severe eosinophilic asthma; SIRIUS, SteroId ReductIon with mepolizUmab Study; SYNAPSE, StudY in NAsal Polyps patients to assess the Safety and Efficacy of mepolizumab
