FIGURE 2.
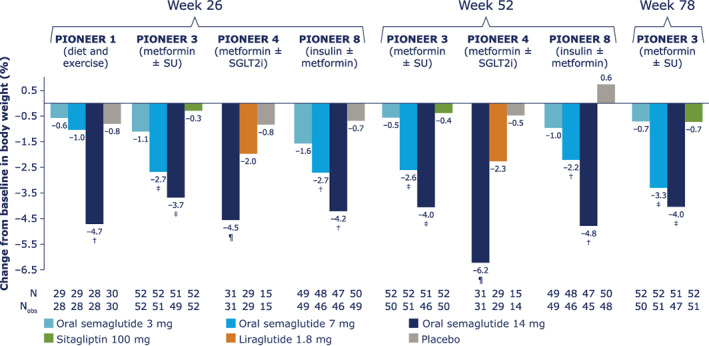
Change from baseline in body weight (%) for Japanese patients in PIONEER 1, 3, 4 and 8. Data from the in‐trial observation period were included in the statistical analysis. The efficacy endpoints were analysed for the treatment policy estimand using a pattern mixture model with multiple imputation to impute missing data for the landmark visits. Imputation of missing data was done using an analysis of covariance (ANCOVA) model within groups defined by randomized treatment and treatment status (on‐treatment without use of rescue medication; premature discontinuation of trial product or initiation rescue medication). After imputation, each of the 1000 imputed, complete datasets were analysed using an ANCOVA model with treatment, strata (PIONEER 3, 4 and 8) and interaction between strata (PIONEER 8) as categorical fixed effects and the baseline value as a covariate. Rubin's rule was used to combine the 1000 analysis results to draw inference. †Upper limit of 95% confidence interval (CI) for estimated treatment difference (ETD) for oral semaglutide 3, 7 and 14 mg versus placebo <0. ‡Upper limit of 95% CI for ETD for oral semaglutide 7 and 14 mg versus sitagliptin <0. ¶Upper limit of 95% CI for ETD for oral semaglutide 14 mg versus liraglutide and placebo <0. N, number of randomized patients contributing to the analyses; Nobs, number of patients with observations; SGLT2i, sodium‐glucose cotransporter‐2 inhibitor; SU, sulphonylurea
