Abstract
Objective
To confirm if mirabegron 50 mg shows efficacy in women with overactive bladder and either urgency urinary incontinence or mixed urinary incontinence versus placebo.
Methods
Post‐hoc analyses were carried out using pooled data from a Japanese phase IIb and a phase III study. The primary efficacy end‐point was baseline to end‐of‐treatment change in the mean number of micturitions/24 h. The secondary end‐points were changes in the mean voided volume/micturition, mean number of urgency and incontinence episodes/24 h, and mean number of nocturia episodes/night. Other end‐points were quality of life and incontinence normalization rates.
Results
Women with urgency urinary incontinence (placebo n = 204, mirabegron n = 214) and mixed urinary incontinence (placebo n = 122, mirabegron n = 139) were included. Change in mean micturitions/24 h at end‐of‐treatment for mirabegron was statistically significant versus placebo in both populations; the effect size increased over time. For all secondary end‐points, median changes for mirabegron were statistically significant versus placebo at end‐of‐treatment, except for nocturia for the urgency urinary incontinence population and urgency for the mixed urinary incontinence population. Mirabegron showed larger improvements versus placebo in all quality‐of‐life domains, except for general health perception in the urgency urinary incontinence population. Incontinence normalization rates for mirabegron were 47.2% and 49.6% in the urgency urinary incontinence and mixed urinary incontinence populations, respectively, versus 42.6% and 39.3% for placebo.
Conclusions
Mirabegron 50 mg significantly improved key overactive bladder symptoms versus placebo in women with urgency urinary incontinence, and it also improved most overactive bladder symptoms, including micturition frequency, in patients with mixed urinary incontinence. These findings support the benefits of using mirabegron in the female overactive bladder wet population.
Keywords: β3‐adrenoreceptor agonist, mirabegron, overactive bladder, urinary incontinence
Abbreviations & Acronyms
- AE
adverse event
- ancova
analysis of covariance
- BMI
body mass index
- CI
confidence interval
- ECG
electrocardiogram
- EOT
end‐of‐treatment
- FAS
full analysis set
- ICS
International Continence Society
- KHQ
King's Health Questionnaire
- LUTS
lower urinary tract symptoms
- MUI
mixed urinary incontinence
- OAB
overactive bladder
- PVR
post‐void residual
- QOL
quality of life
- SAF
safety analysis set
- SD
standard deviation
- SUI
stress urinary incontinence
- TEAE
treatment‐emergent adverse event
- UUI
urgency urinary incontinence
Introduction
Female urinary incontinence, OAB and other LUTS are highly prevalent conditions with a profound influence on well‐being and QOL. 1 , 2 , 3 , 4 , 5 In Japan, a 2002 survey estimated OAB prevalence to be 12.4% (11% in women, 14% in men), increasing with age. 6 In a large‐scale nationwide epidemiological survey of randomly selected Japanese men and women aged ≥40 years, 15% experienced a negative impact on daily life from LUTS, although just 3% of the whole sample sought medical care. 7
Incontinence is among the most troublesome of the OAB symptoms and is associated with the most significant impairment on QOL. 8 The ICS identifies several subtypes of urinary incontinence, including UUI (involuntary loss of urine associated with urgency), SUI (involuntary loss of urine on effort or physical exertion, or on sneezing or coughing) and MUI (involuntary loss of urine associated with urgency and with exertion, effort, sneezing or coughing). Based on the current ICS definition of OAB, all patients with UUI have OAB, whereas not all patients with OAB have UUI. 9 In the 2002 Japanese survey, prevalence rates for OAB were 6.4% with UUI (≥1 episode/week) and 6.0% without UUI. 6
Mirabegron is a β3‐adrenoreceptor agonist that causes detrusor muscle relaxation, thereby inhibiting micturition and increasing urine storage in the bladder. It represents an alternative to antimuscarinics for OAB treatment, with a potentially favorable benefit‐to‐risk ratio. 10 , 11 In a Japanese phase IIb dose‐finding study in which >80% of participants were women (study CL‐045, ClinicalTrials.gov NCT00527033), mirabegron at daily doses of 25, 50 and 100 mg showed significant improvements versus placebo in mean change from baseline in the number of micturitions/24 h. 12 Significant improvements were also observed in incontinence, urgency incontinence, mean voided volume/micturition and three of nine domains from the KHQ (incontinence impact, physical limitations and severity measures) at each mirabegron dose. Urgency episodes decreased and mean voided volume increased, both dose‐dependently, with no clinically significant safety concerns. In a subsequent Japanese phase III study in which >82% of participants were women (CL‐048, ClinicalTrials.gov NCT00966004), mirabegron 50 mg was selected to assess efficacy (superiority) and safety versus both placebo‐ and tolterodine‐treated groups (without testing). 13 The results showed that mirabegron was significantly superior to placebo in terms of mean change from baseline in the number of micturitions/24 h and in mean change from baseline in the number of urgency episodes/24 h, incontinence episodes/24 h, urgency incontinence episodes/24 h and voided volume/micturition. The incidence of AEs in the mirabegron group was similar to that in the placebo group. Most AEs were mild and none were severe.
There is sparse evidence for the efficacy of mirabegron in incontinent OAB (OAB wet) populations. 14 The current post‐hoc analysis used data for women with incontinence extracted from the two pivotal placebo‐controlled Japanese studies; 12 , 13 the objectives were to confirm these data, which suggest that mirabegron 50 mg shows clinical improvement in OAB‐related symptoms and QOL scores in women with UUI or MUI versus placebo, and to examine potential factors that affect efficacy.
Methods
Post‐hoc analyses were conducted using pooled data from a phase IIb and a phase III study carried out in Japan. The protocol for each study was approved by the respective institutional review boards and the studies were carried out in accordance with the Declaration of Helsinki. The full methodology for these pivotal studies is described in the primary publications; 12 , 13 both were 12‐week, multicenter, double‐blind, double‐dummy, parallel‐group comparative studies in male and female patients with OAB symptoms for ≥24 weeks, eight or more micturitions/24 h on average and at least one episode of urgency and/or urgency incontinence/24 h. Therefore, patients may or may not have been incontinent. The inclusion and exclusion criteria were similar between the studies.
All patients in the post‐hoc analysis had at least one episode of incontinence/24 h at baseline, were female, had received either mirabegron 50 mg or placebo and were designated as belonging to either the UUI or MUI population on the basis of investigator diagnosis at study enrolment. The methods regarding the FAS, QOL analysis set and SAF were the same as those used in the individual studies. 12 , 13 The primary efficacy end‐point for the post‐hoc analysis was change from baseline to EOT in mean number of micturitions/24 h. Median change in the mean number of micturitions/24 h was considered the treatment effect within each treatment group. Secondary end‐points were change from baseline to EOT in mean voided volume/micturition, mean number of urgency episodes/24 h, mean number of incontinence episodes/24 h and mean number of nocturia episodes/night. Other end‐points were mean change from baseline to EOT in the individual domains of the KHQ that measure aspects of QOL and incontinence normalization rates (complete absence of symptoms at EOT that were present at baseline, i.e. zero incontinence episodes).
For the exploratory analysis, multiple regression analysis was used to search for factors with a large impact on the primary end‐point. Potential factors included age, treatment (mirabegron vs placebo), mean number of micturitions/24 h at baseline, mean number of urgency episodes/24 h at baseline, voided volume/micturition at baseline, mean number of incontinence episodes/24 h at baseline, nocturia episodes/night at baseline (yes or no), BMI, previous medication for OAB (yes or no), previous anticholinergic medication, OAB duration (months) and KHQ total QOL score at baseline. The final model was selected from a clinical point of view, referring to Akaike's information criterion as a selection indicator.
Safety was evaluated on the basis of TEAEs, vital signs (systolic and diastolic blood pressure, and pulse rate), 12‐lead ECG (including QTc interval) and PVR urine volume.
Statistical analysis
Statistical analyses for the primary and secondary end‐points were carried out using data at EOT; no statistical comparisons were conducted on data at weeks 4 and 8. All analyses for efficacy end‐points, except for the KHQ, were carried out using the FAS‐MUI (women with ≥1 mean urinary incontinence episode/24 h at baseline and diagnosed by the investigator as belonging to the MUI population) and the FAS‐UUI (women with ≥1 mean urinary incontinence episode/24 h at baseline and diagnosed by the investigator as belonging to the UUI population). For the KHQ (QOL score), QOL analysis set‐MUI and QOL analysis set‐UUI were analyzed. The safety evaluation was carried out using the SAF‐MUI and SAF‐UUI.
For continuous variables, summary statistics included the number of patients (n), mean, SD, median, and Q1 and Q3, which represent the 25th and 75th percentiles of the population. For efficacy data, the 10th and 90th percentiles (minimum and maximum) were also included. Categorical data were presented as frequencies and percentages. All statistical comparisons were made using two‐sided tests at the α = 0.05 significance level unless stated otherwise. All null hypotheses were of no treatment difference, all alternative hypotheses were two‐sided. All data processing, summarization, and analyses were carried out using SAS version 9.4 (SAS, Cary, NC, USA) or higher on Linux.
For the primary end‐point, a multiple regression analysis was used to search for factors with a large impact from age, treatment, mean number of micturitions/24 h (baseline), mean number of urgency episodes/24 h (baseline), voided volume per micturition (baseline), mean number of incontinence/24 h (baseline), night time voiding (yes/no), BMI, previous medication for OAB (yes/no), previous anticholinergic medication, OAB duration (days) and total KHQ (QOL score). The primary analysis for change in mean number of micturitions/24 h was carried out using the rank ancova model. Because a stratified rank ancova only provides P‐values for group comparisons, an estimate of the difference in median change in mean number of micturitions/24 h and a 95% CI around that estimate was calculated using the Hodges–Lehmann method. This was also used to obtain an estimate of the median (and 95% CI) change in mean number of micturitions/24 h within treatment groups.
Results
Study population
A total of 918 women were randomized (448 in the placebo group, 470 in the mirabegron 50 mg group) in the original trials. For the UUI population, 418 women were included in the FAS (204 placebo, 214 mirabegron 50 mg) and 572 were included in the SAF (287 placebo, 285 mirabegron 50 mg). For the MUI population, 261 women were included in the FAS (122 placebo, 139 mirabegron 50 mg) and 341 were included in the SAF (157 placebo, 184 mirabegron 50 mg).
Patient demographics and characteristics at baseline were very similar in the mirabegron 50 mg and placebo groups in both the UUI and MUI populations (Table 1). The mean age was 58 years for the UUI population and 59 years for the MUI population. In the UUI population, 40% of the placebo group and 38% of the mirabegron 50 mg group were aged ≥65 years; in the MUI population, 36% and 34% of the placebo and mirabegron groups, respectively, were aged ≥65 years. The median duration of OAB for the UUI population was 55.0 months for the placebo and mirabegron 50 mg groups, and for the MUI population was 52.5 months for the placebo group and 57.0 months for the mirabegron 50 mg group. At baseline, the number of micturitions/24 h was comparable for the mirabegron 50 mg and placebo groups (mean of 11 episodes/24 h in all populations and treatment groups). However, the mean number of urgency episodes/24 h was slightly less in the MUI population than in the UUI population. Median PVR urine volume for the UUI population was 4.80 mL for the placebo group and 2.55 mL for the mirabegron 50 mg group, and for the MUI population was 0.65 mL for the placebo group and 4.70 mL for the mirabegron 50 mg group.
Table 1.
Baseline characteristics, by population (FAS)
| UUI population | MUI population | |||
|---|---|---|---|---|
| Placebo (n = 204) | Mirabegron 50 mg (n = 214) | Placebo (n = 122) | Mirabegron 50 mg (n = 139) | |
| Mean age, years (SD) | 58.3 (13.4) | 57.9 (14.0) | 59.4 (11.3) | 58.8 (12.3) |
| Median | 60.0 | 59.5 | 61.0 | 60.0 |
| Q1–Q3 | 47.0–68.5 | 48.0–69.0 | 51.0–67.0 | 48.0–69.0 |
| Age group, n (%) | ||||
| <65 years | 122 (59.8) | 133 (62.1) | 78 (63.9) | 92 (66.2) |
| ≥65 years | 82 (40.2) | 81 (37.9) | 44 (36.1) | 47 (33.8) |
| Mean weight, kg (SD) | 53.51 (7.88) | 53.74 (8.85) | 55.28 (9.27) | 56.17 (10.19) |
| Median | 53.00 | 52.40 | 54.45 | 54.80 |
| Q1–Q3 | 48.95–57.0 | 47.90–58.0 | 49.00–59.50 | 49.00–61.50 |
| Mean height, cm (SD) | 155.25 (6.40) | 155.22 (6.17) | 154.11 (6.30) | 153.96 (5.78) |
| Median | 155.40 | 155.00 | 154.00 | 154.00 |
| Q1–Q3 | 151.0–159.75 | 151.0–159.40 | 150.0–158.0 | 150.0–158.0 |
| Mean duration of illness, months† (SD) | 81.9 (95.6) | 74.7 (69.5) | 68.9 (53.0) | 83.2 (75.4) |
| Median | 55.0 | 55.0 | 52.5 | 57.0 |
| Q1–Q3 | 33.0–93.0 | 33.0–93.0 | 32.5–95.0 | 34.0–104.0 |
| OAB severity (mean no. micturitions/24 h), n (%) | ||||
| <10 | 65 (31.9) | 64 (29.9) | 39 (32.0) | 46 (33.1) |
| ≥10 to <15 | 127 (62.3) | 134 (62.6) | 78 (63.9) | 75 (54.0) |
| ≥15 | 12 (5.9) | 16 (7.5) | 5 (4.1) | 18 (12.9) |
| Mean PVR urine volume, mL (SD) | 9.68 (13.54) | 8.20 (14.01) | 7.05 (14.22) | 10.14 (13.85) |
| Median | 4.80 | 2.55 | 0.65 | 4.70 |
| Q1–Q3 | 0–14.00 | 0–10.20 | 0–8.40 | 0–14.50 |
| Medical history, n (%) | ||||
| No | 177 (86.8) | 180 (84.1) | 101 (82.8) | 116 (83.5) |
| Yes | 27 (13.2) | 34 (15.9) | 21 (17.2) | 23 (16.5) |
| Complications, n (%) | ||||
| No | 52 (25.5) | 60 (28.0) | 27 (22.1) | 31 (22.3) |
| Yes | 152 (74.5) | 154 (72.0) | 95 (77.9) | 108 (77.7) |
| Previous medications, n (%) | ||||
| No | 76 (37.3) | 78 (36.4) | 49 (40.2) | 60 (43.2) |
| Yes | 128 (62.7) | 136 (63.6) | 73 (59.8) | 79 (56.8) |
| Concomitant medications, n (%) | ||||
| No | 61 (29.9) | 62 (29.0) | 31 (25.4) | 38 (27.3) |
| Yes | 143 (70.1) | 152 (71.0) | 91 (74.6) | 101 (72.7) |
| Mean no. micturitions/24 h (SD) | 11.21 (2.47) | 11.48 (2.67) | 11.10 (2.16) | 11.49 (2.76) |
| Min–max | 8.0–21.3 | 7.7–23.3 | 8.0–18.0 | 8.0–21.3 |
| Median | 11.00 | 11.00 | 10.67 | 10.67 |
| Q1–Q3 | 9.33–12.50 | 9.33–13.00 | 9.67–12.33 | 9.33–13.00 |
| Mean no. urgency episodes/24 h (SD) | 4.85 (2.80) | 5.11 (3.09) | 4.38 (2.67) | 4.89 (3.10) |
| Min–max | 1.0–14.3 | 1.0–15.7 | 1.0–13.7 | 1.0–16.0 |
| Median | 4.33 | 4.33 | 3.67 | 4.33 |
| Q1–Q3 | 2.67–6.67 | 2.67–6.33 | 2.67–5.67 | 2.67–6.33 |
| Mean no. incontinence episodes/24 h (SD) | 1.88 (1.71) | 2.07 (1.86) | 1.86 (1.79) | 2.24 (2.64) |
| Min–max | 0.3–9.7 | 0.3–12.7 | 0.3–12.0 | 0.3–21.0 |
| Median | 1.33 | 1.67 | 1.67 | 1.33 |
| Q1–Q3 | 0.67–2.33 | 1.00–2.67 | 0.67–2.33 | 0.67–3.00 |
| Mean no. urge incontinence episodes/24 h (SD [n]) | 1.67 (1.37) [196] | 1.85 (1.70) [204] | 1.54 (1.39) [118] | 2.02 (2.29) [135] |
| Min–max | 0.3–7.7 | 0.3–12.7 | 0.3–6.7 | 0.3–15.7 |
| Median | 1.33 | 1.33 | 1.17 | 1.33 |
| Q1–Q3 | 0.67–2.33 | 0.67–2.33 | 0.67–2.00 | 0.67–2.67 |
| Mean voided volume/micturition, mL (SD [n]) | 149.4 (43.9) [203] | 148.3 (45.0) [214] | 148.5 (43.1) [121] | 153.6 (51.1) [138] |
| Min–max | 56.46–282.80 | 50.00–276.00 | 60.43–275.00 | 45.67–332.08 |
| Median | 143.261 | 141.584 | 140.769 | 148.267 |
| Q1–Q3 | 117.742–176.875 | 114.211–180.789 | 117.000–179.531 | 120.962–185.000 |
| Mean no. nocturia episodes (SD [n]) | 1.76 (1.19) [175] | 1.71 (0.97) [197] | 1.76 (1.12) [108] | 1.61 (1.03) [119] |
| Min–max | 0.5–6.5 | 0.5–5.0 | 0.5–5.5 | 0.5–5.0 |
| Median | 1.50 | 1.50 | 1.50 | 1.50 |
| Q1–Q3 | 1.00–2.00 | 1.00–2.50 | 1.0–2.50 | 1.0–2.00 |
| QOL analysis set, mean (SD) of KHQ domain score | n = 201 | n = 211 | n = 121 | n = 136 |
| D1: general health perception | 32.8 (18.6) | 31.2 (19.3) | 33.5 (19.2) | 32.5 (17.0) |
| D2: incontinence impact | 52.9 (28.2) | 51.8 (26.6) | 44.9 (24.6) | 46.8 (29.4) |
| D3: role limitations | 39.4 (26.1) | 36.2 (26.0) | 37.5 (23.6) | 38.5 (26.2) |
| D4: physical limitations | 40.2 (27.5) | 38.1 (27.8) | 42.7 (25.4) | 41.7 (29.4) |
| D5: social limitations | 21.1 (23.5) | 20.8 (23.6) | 20.4 (22.2) | 21.2 (23.8) |
| D6: personal relationships [n] | 9.4 (17.9) [153] | 11.7 (20.4) [157] | 6.9 (13.3) [92] | 10.5 (21.4) [106] |
| D7: emotions | 42.7 (28.3) | 39.7 (25.6) | 41.2 (26.2) | 37.2 (26.4) |
| D8: sleep/energy | 31.8 (26.2) | 30.9 (23.4) | 31.5 (25.7) | 27.5 (24.8) |
| D9: severity measures | 36.4 (19.9) | 36.7 (18.2) | 36.3 (18.2) | 37.6 (21.9) |
For the UUI population, n = 203 for placebo, n = 209 for mirabegron 50 mg. For the MUI population, n = 120 for placebo, n = 134 for mirabegron 50 mg.
Primary end‐point
Estimates for median change from baseline to EOT in the number of micturitions/24 h and the treatment difference in median change are shown in Figure 1. Change in the mean number of micturitions/24 h at EOT for mirabegron 50 mg was statistically significant versus placebo in both the MUI and UUI populations. Although no statistical comparisons were carried out at weeks 4 and 8, numerical improvements were seen over time at all timepoints.
Fig. 1.
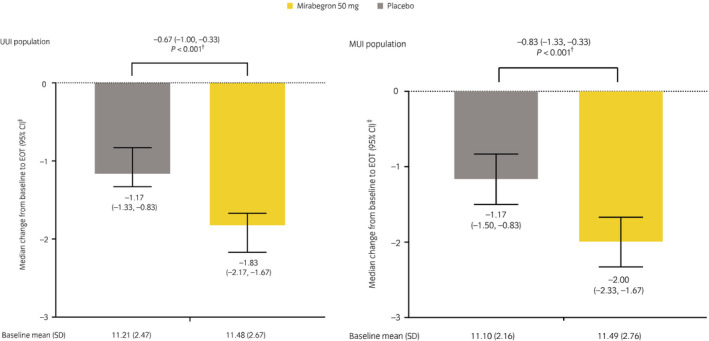
Change from baseline to EOT in the number of micturitions/24 h in the UUI population and the MUI population (FAS). †A stratified rank ancova was used to compare the median change between placebo and mirabegron 50 mg. ‡The Hodges–Lehmann method was used to obtain an estimate for the median (and 95% CI) change.
Secondary end‐points
For the UUI population, the median changes for mirabegron 50 mg were statistically significant versus placebo at EOT for all of the secondary end‐points, except for nocturia episodes (Table 2). With the exception of the results for nocturia, numerical improvements were seen over time at all timepoints. For the MUI population, the median changes for mirabegron 50 mg were statistically significant versus placebo at EOT for all of the secondary end‐points, except for the number of urgency episodes/24 h, although the reduction in the number of urgency episodes was numerically greater for mirabegron 50 mg versus placebo. Numerical improvements were seen over time at all timepoints.
Table 2.
Estimates for the change from baseline in secondary end‐points, by population (FAS)
| UUI population | MUI population | |||
|---|---|---|---|---|
| Placebo (n = 204) | Mirabegron 50 mg (n = 214) | Placebo (n = 122) | Mirabegron 50 mg (n = 139) | |
| Urgency episodes/24 h | ||||
| Median value at baseline (Q1–Q3) | 4.33 (2.67–6.67) | 4.33 (2.67–6.33) | 3.67 (2.67–5.67) | 4.33 (2.67–6.33) |
| Estimate of the median change (95% two‐sided CI)† | −1.83 (−2.17, −1.5) | −2.50 (−2.83, −2.17) | −1.67 (−2.17, −1.17) | −2.17 (−2.50, −1.83) |
| Difference in median change versus placebo (95% two‐sided CI)† | −0.67 (−1.00, −0.33) | −0.50 (−1.00, −0.00) | ||
| Two‐sided P‐value‡ | 0.002 | 0.266 | ||
| Incontinence episodes/24 h | ||||
| Median value at baseline (Q1–Q3) | 1.33 (0.67–2.33) | 1.67 (1.00–2.67) | 1.67 (0.67–2.33) | 1.33 (0.67–3.00) |
| Estimate of the median change (95% two‐sided CI)† | −0.67 (−0.83, −0.50) | −1.00 (−1.17, −1.00) | −0.67 (−0.83, −0.50) | −1.17 (−1.33, −1.00) |
| Difference in median change versus placebo (95% two‐sided CI)† | −0.33 (−0.67, 0.00) | −0.50 (−0.67, −0.33) | ||
| Two‐sided P‐value‡ | 0.002 | <0.001 | ||
| Mean voided volume/micturition | ||||
| Median value at baseline (Q1–Q3) | 143.261 (117.742–176.875) | 141.584 (114.211–180.789) | 140.769 (117.000–179.531) | 148.267 (120.962–185.000) |
| Estimate of the median change (95% two‐sided CI)† | 6.303 (2.244–10.439) | 25.619 (21.144–30.070) | 14.578 (8.301–20.685) | 24.839 (19.476–30.837) |
| Difference in median change versus placebo (95% two‐sided CI)† | 19.203 (13.121–25.285) | 10.973 (3.363–18.583) | ||
| Two‐sided P‐value‡ | <0.001 | 0.005 | ||
| Nocturia episodes/night | ||||
| Median value at baseline (Q1–Q3) | 1.50 (1.00–2.00) | 1.50 (1.00–2.50) | 1.50 (1.00–2.50) | 1.50 (1.00–2.00) |
| Estimate of the median change (95% two‐sided CI)† | −0.25 (−0.50, −0.25) | −0.50 (−0.50, −0.25) | −0.25 (−0.50, 0.00) | −0.50 (−0.50, −0.25) |
| Difference in median change versus placebo (95% two‐sided CI)† | 0.00 (0.00, 0.00) | −0.25 (−0.50, 0.00) | ||
| Two‐sided P‐value‡ | 0.588 | 0.030 | ||
The Hodges–Lehmann method was used to obtain an estimate in the median (and 95% CI) change.
Ranked ancova (vs placebo).
Other end‐points
Incontinence normalization rates for the mirabegron 50 mg group were 47.2% and 49.6% in the UUI and MUI populations, respectively, whereas those for placebo were 42.6% and 39.3%. The change from baseline to EOT in KHQ domain scores, by population, is shown in Figure 2. The mirabegron 50 mg group showed larger improvements versus placebo in all domains of the KHQ, except for general health perception in the UUI population.
Fig. 2.
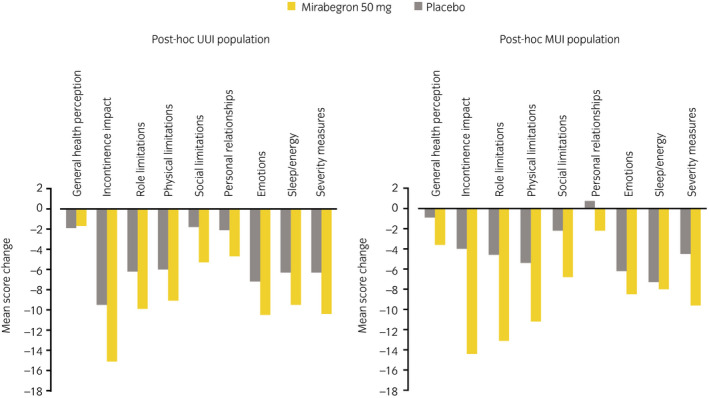
Change from baseline to EOT in KHQ domain score, by population (QOL analysis set).
Exploratory end‐points
Multiple regression analysis suggested that the factors positively affecting the primary end‐point were the mean number of micturitions/24 h at baseline and treatment with mirabegron 50 mg in both the UUI and MUI populations (Table S1). Previous medication for OAB was the only factor with an adverse effect on the primary end‐point.
Safety
The overall incidence of treatment‐related AEs (approximately 23–26%) was similar in the mirabegron 50 mg and placebo groups for both the UUI and MUI populations. Common treatment‐related AEs (incidence of ≥2% in any treatment group) are shown in Table 3. The incidence of dry mouth and constipation, which are commonly associated with antimuscarinic agents, was similar between the mirabegron 50 mg and placebo groups. Vital signs (pulse rate and blood pressure) and ECG findings in the mirabegron 50 mg group were generally similar to those in the placebo group for both populations. There were no changes of note in PVR urine volume for either population.
Table 3.
Summary of treatment‐related AEs occurring in ≥2% of patients in any treatment group, by population (SAF)
| AEs, n (%) | UUI population | MUI population | ||
|---|---|---|---|---|
| Placebo (n = 287) | Mirabegron 50 mg (n = 285) | Placebo (n = 157) | Mirabegron 50 mg (n = 184) | |
| Total TEAEs | 214 (74.6) | 215 (75.4) | 126 (80.3) | 145 (78.8) |
| Treatment‐related AEs | 65 (22.6) | 71 (24.9) | 39 (24.8) | 47 (25.5) |
| Dry mouth | 6 (2.1) | 4 (1.4) | 5 (3.2) | 6 (3.3) |
| Constipation | 4 (1.4) | 11 (3.9) | 5 (3.2) | 5 (2.7) |
| Gamma‐glutamyltransferase increased | 6 (2.1) | 15 (5.3) | 5 (3.2) | 10 (5.4) |
| Alanine aminotransferase increased | 5 (1.7) | 4 (1.4) | 0 | 5 (2.7) |
| Blood creatine phosphokinase increased | 13 (4.5) | 11 (3.9) | 7 (4.5) | 5 (2.7) |
| Protein urine present | 3 (1.0) | 6 (2.1) | 1 (0.6) | 0 |
| Aspartate aminotransferase increased | 5 (1.7) | 2 (0.7) | 3 (1.9) | 3 (1.6) |
| Blood alkaline phosphatase increased | 3 (1.0) | 12 (4.2) | 5 (3.2) | 3 (1.6) |
| Treatment‐related serious AEs | 2 (0.7) | 0 | 0 | 1 (0.5) |
| Treatment‐related AEs leading to withdrawal of treatment | 2 (0.7) | 6 (2.1) | 3 (1.9) | 6 (3.3) |
Discussion
To confirm the clinical utility of mirabegron 50 mg for women with OAB and UUI or MUI, we carried out a post‐hoc analysis of datasets extracted from a phase IIb and a phase III study performed in Japan. 12 , 13 The results from the present post‐hoc analysis showed that mirabegron 50 mg significantly improved all key symptoms in women with OAB and UUI compared with placebo, with an increasing effect size over time. One exception was the lack of a statistically significant effect of mirabegron on nocturia; however, in addition to the relatively low number of nocturia episodes at baseline, multiple factors, including nocturnal polyuria and sleep disorder, might have contributed to the lack of difference versus placebo. A similar lack of statistically significant effect on nocturia episodes has been shown in previous mirabegron studies, 13 and in several clinical trials for antimuscarinics in patients with OAB. 15 , 16 , 17
Furthermore, compared with placebo, mirabegron 50 mg resulted in improvement of key OAB symptoms in the MUI population, including statistically significant improvements in micturition frequency, although not in urgency episodes. It is possible that the study power was insufficient to detect the effect on urgency in this post‐hoc analysis, because there were just 122 patients in the placebo group and 139 in the mirabegron 50 mg group versus 211 and 208 patients, respectively, in the phase IIb study, 12 and 368 and 369 patients, respectively, in the phase III study. 13 In terms of absolute differences, there was a median of 0.50 fewer urgency episodes for mirabegron versus placebo for the post‐hoc analysis (95% CI −1.00, −0.00). In comparison, there was a mean of 0.41 fewer urgency episodes with mirabegron 50 mg versus placebo for the phase IIb study, 12 and a mean of 0.48 fewer urgency episodes with mirabegron 50 mg versus placebo for the phase III study. 13
It is unclear whether mirabegron improved SUI symptoms in the MUI population in the current post‐hoc analysis. However, a hypothesis for the positive effect of mirabegron on micturition frequency in the MUI population is that even when abdominal pressure is increased (e.g. when standing), mirabegron might not induce a transient excessive increase in intravesical pressure, because of improved bladder compliance. 18 , 19 An alternative hypothesis is that by improving bladder compliance, mirabegron might suppress the sensation of bladder filling experienced in women with SUI. As a result, women are less anxious about being incontinent and do not resort to preventive voiding, which means that the number of micturitions is decreased. Mirabegron might, therefore, be the preferred drug for patients with MUI‐type OAB. However, regarding the direct effect on SUI, randomized controlled trials in patients with SUI are recommended to provide additional data.
Vibegron, approved in Japan in 2018, is another selective β3‐adrenergic receptor agonist. Although vibegron is associated with significant improvements versus placebo in micturition, UUI and maximum voided volume in patients with OAB symptoms with or without incontinence at baseline, there are currently no data in patients with OAB who have MUI. 20 , 21
As noted previously, incontinence is a particularly bothersome OAB symptom, associated with significant impairment in QOL. 8 In both the UUI and MUI populations, treatment with mirabegron 50 mg resulted in a higher frequency of responders in terms of incontinence normalization rates.
The current post‐hoc results extend and support results from a subgroup analysis of patients with UUI or MUI from studies of solifenacin reported by Kelleher et al. 22 A greater proportion of patients receiving solifenacin achieved resolution of incontinence in both the MUI and UUI groups (MUI: 5 mg = 43%, 10 mg = 49%; UUI: 5 mg = 55%, 10 mg = 54%) compared with patients receiving placebo (MUI 33%, UUI 35%).
The current post‐hoc results also extend and support results from a post‐hoc analysis of pooled data from male and female patients with OAB wet who participated in three randomized, double‐blind, placebo‐controlled, 12‐week, phase III studies reported by Chapple et al. 14 In the Chapple analysis, patients receiving mirabegron 50 mg (n = 862) showed statistically significant improvements from baseline to final visit versus placebo (n = 878) in the mean number of incontinence episodes, micturitions and urgency episodes/24 h, and in the mean voided volume/micturition in the pooled incontinent population. This was also the case for subgroups of patients stratified by the severity of incontinence at baseline. Furthermore, in the current post‐hoc analysis, the mirabegron 50 mg group showed larger improvements versus placebo in all but one domain of the KHQ in the UUI population. The KHQ is considered a reliable and valid tool for assessing QOL in patients with OAB and incontinence in Japan. 23
The multiple regression analysis carried out as an exploratory analysis suggested that some baseline factors might affect the efficacy of mirabegron 50 mg in both populations. The primary end‐point was negatively affected by previous OAB medication, suggesting these patients might be refractory to treatment. In a previous analysis of two 12‐week, double‐blind, head‐to‐head studies, adults with OAB symptoms plus at least one UUI episode and eight or more micturitions/24 h at baseline were randomized to fesoterodine 8 mg, extended‐release tolterodine 4 mg or placebo; lack of previous antimuscarinic treatment, as well as female sex, younger age and shorter duration of OAB, were common predictors of larger changes in outcomes from baseline to week 12. 24 In addition, although higher BMI was associated with increased incidence of SUI due to increased intra‐abdominal and intravesical pressure, 25 , 26 BMI was not identified as a factor adversely affecting the efficacy of mirabegron in this post‐hoc analysis, or in a study of 169 women prescribed mirabegron 50 mg. 27 Similarly, in the analysis by Herschorn et al., 24 BMI was not found to be associated with treatment response to fesoterodine or extended‐release tolterodine.
Limitations of the present study were the younger mean age in this post‐hoc analysis (~58–59 years) in comparison with a mean age of >70 years for patients with OAB in Japan in a real‐world clinical setting. 28 In addition, if incontinence occurred during the study period, the specific type of incontinence (UUI, SUI or other) was not identified. Furthermore, because they might have a complicated pathology or symptoms related to benign prostatic hyperplasia, male patients were excluded from this analysis. Therefore, additional evidence in men is required.
There are no apparent differences in the safety profile between the post‐hoc analysis and the original studies. 12 , 13 In the post‐hoc analysis, the overall incidence of treatment‐related AEs was similar in the mirabegron 50 mg and placebo groups for both populations, with the incidence of anticholinergic AEs, and vital signs and ECG findings also similar between the mirabegron and placebo groups for both populations.
In conclusion, the results from the present post‐hoc analysis showed that mirabegron 50 mg significantly improved most key OAB symptoms versus placebo in female patients with OAB who had UUI. In addition, versus placebo, mirabegron 50 mg resulted in improvement of most OAB symptoms, including micturition frequency, in female patients with OAB who had MUI. These results support the benefits of mirabegron in the female OAB wet population.
Conflict of interest
Satoru Takahashi is a consultant for Astellas. Yuji Mishima, Kentaro Kuroishi and Masashi Ukai are employees of Astellas Pharma.
Approval of the research protocol
The protocol for each study was approved by the respective Institutional Review Boards and the studies were conducted in accordance with the Declaration of Helsinki.
Informed consent
All patients provided written informed consent before the start of the placebo run‐in period.
Registration of the studies
Study CL‐045: NCT00527033 (ClinicalTrials.gov). Study CL‐048: NCT00966004 (ClinicalTrials.gov).
Animal studies
N/A.
Supporting information
Table S1. Multiple regression analysis, by population (FAS‐UUI and FAS‐MUI analysis sets).
Acknowledgments
This study was funded by Astellas Pharma Inc. Medical writing support was provided by Sue Cooper, CMPP, of Envision Scientific Solutions and funded by Astellas Pharma Inc. The authors thank the 045 and 048 study investigators, and all patients and their parents/legal representatives who took part in the studies.
Data availability statement
Researchers may request access to anonymized participant‐level data, trial‐level data and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.
References
- 1. Coyne KS, Sexton CC, Irwin DE, Kopp ZS, Kelleher CJ, Milsom I. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well‐being in men and women: results from the EPIC study. BJU Int. 2008; 101: 1388–95. [DOI] [PubMed] [Google Scholar]
- 2. Irwin DE, Milsom I, Kopp Z, Abrams P, Cardozo L. Impact of overactive bladder symptoms on employment, social interactions and emotional well‐being in six European countries. BJU Int. 2006; 97: 96–100. [DOI] [PubMed] [Google Scholar]
- 3. Papanicolaou S, Hunskaar S, Lose G, Sykes D. Assessment of bothersomeness and impact on quality of life of urinary incontinence in women in France, Germany, Spain and the UK. BJU Int. 2005; 96: 831–8. [DOI] [PubMed] [Google Scholar]
- 4. Milsom I, Kaplan SA, Coyne KS, Sexton CC, Kopp ZS. Effect of bothersome overactive bladder symptoms on health‐related quality of life, anxiety, depression, and treatment seeking in the United States: results from EpiLUTS. Urology 2012; 80: 90–6. [DOI] [PubMed] [Google Scholar]
- 5. Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Economic burden of urgency urinary incontinence in the United States: a systematic review. J. Manag. Care Pharm. 2014; 20: 130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Homma Y, Yamaguchi O, Hayashi K, Neurogenic Bladder Society Committee . An epidemiological survey of overactive bladder symptoms in Japan. BJU Int. 2005; 96: 1314–8. [DOI] [PubMed] [Google Scholar]
- 7. Homma Y, Yamaguchi O, Hayashi K, Neurogenic Bladder Society Committee . Epidemiologic survey of lower urinary tract symptoms in Japan. Urology 2006; 68: 560–4. [DOI] [PubMed] [Google Scholar]
- 8. Coyne KS, Wein A, Nicholson S, Kvasz M, Chen CI, Milsom I. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int. J. Clin. Pract. 2013; 67: 1015–33. [DOI] [PubMed] [Google Scholar]
- 9. Abrams P, Cardozo L, Fall M et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub‐committee of the International Continence Society. Urology 2003; 61: 37–49. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida M, Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder aged ≥75 years: analysis of a Japanese post‐marketing study. Low. Urin. Tract Symptoms 2019; 11: 30–8. [DOI] [PubMed] [Google Scholar]
- 11. Chapple CR, Siddiqui E. Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor‐responder populations, and patients with OAB in Asia. Expert Rev. Clin. Pharmacol. 2017; 10: 131–51. [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi O, Marui E, Igawa Y et al. Efficacy and safety of the selective β3 ‐adrenoceptor agonist mirabegron in Japanese patients with overactive bladder: a randomized, double‐blind, placebo‐controlled, dose‐finding study. Low. Urin. Tract Symptoms 2015; 7: 84–92. [DOI] [PubMed] [Google Scholar]
- 13. Yamaguchi O, Marui E, Kakizaki H et al. Phase III, randomised, double‐blind, placebo‐controlled study of the β3‐adrenoceptor agonist mirabegron, 50 mg once daily, in Japanese patients with overactive bladder. BJU Int. 2014; 113: 951–60. [DOI] [PubMed] [Google Scholar]
- 14. Chapple C, Khullar V, Nitti VW et al. Efficacy of the β3‐adrenoceptor agonist mirabegron for the treatment of overactive bladder by severity of incontinence at baseline: a post hoc analysis of pooled data from three randomised phase 3 trials. Eur. Urol. 2015; 67: 11–4. [DOI] [PubMed] [Google Scholar]
- 15. Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z. 037 Study Group. Nighttime dosing with tolterodine reduces overactive bladder‐related nocturnal micturitions in patients with overactive bladder and nocturia. Urology 2006; 67: 731–6. [DOI] [PubMed] [Google Scholar]
- 16. Dmochowski RR, Peters KM, Morrow JD et al. Randomized, double‐blind, placebo‐controlled trial of flexible‐dose fesoterodine in subjects with overactive bladder. Urology 2010; 75: 62–8. [DOI] [PubMed] [Google Scholar]
- 17. Herschorn S, Swift S, Guan Z et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head‐to‐head placebo‐controlled trial. BJU Int. 2010; 105: 58–66. [DOI] [PubMed] [Google Scholar]
- 18. Kamei J, Furuta A, Akiyama Y et al. Video‐urodynamic effects of mirabegron, a β3 ‐adrenoceptor agonist, in patients with low‐compliance bladder. Int. J. Urol. 2015; 22: 956–61. [DOI] [PubMed] [Google Scholar]
- 19. Matsukawa Y, Takai S, Funahashi Y, Yamamoto T, Gotoh M. Urodynamic evaluation of the efficacy of mirabegron on storage and voiding functions in women with overactive bladder. Urology 2015; 85: 786–90. [DOI] [PubMed] [Google Scholar]
- 20. Shi H, Chen H, Zhang Y, Cui Y. The efficacy and safety of vibegron in treating overactive bladder: a systematic review and pooled analysis of randomized controlled trials. Neurourol. Urodyn. 2020; 39: 1255–63. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida M, Takeda M, Gotoh M et al. Efficacy of vibegron, a novel β3‐adrenoreceptor agonist, on severe urgency urinary incontinence related to overactive bladder: post hoc analysis of a randomized, placebo‐controlled, double‐blind, comparative phase 3 study. BJU Int. 2020; 125: 709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelleher C, Cardozo L, Kobashi K, Lucente V. Solifenacin: as effective in mixed urinary incontinence as in urge urinary incontinence. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006; 17: 382–8. [DOI] [PubMed] [Google Scholar]
- 23. Uemura S, Homma Y. Reliability and validity of King's Health Questionnaire in patients with symptoms of overactive bladder with urge incontinence in Japan. Neurourol. Urodyn. 2004; 23: 94–100. [DOI] [PubMed] [Google Scholar]
- 24. Herschorn S, Kaplan SA, Sun F, Ntanios F. Do patient characteristics predict responsiveness to treatment of overactive bladder with antimuscarinic agents? Urology 2014; 83: 1023–9. [DOI] [PubMed] [Google Scholar]
- 25. Dwyer PL, Lee ET, Hay DM. Obesity and urinary incontinence in women. Br. J. Obstet. Gynaecol. 1988; 95: 91–6. [DOI] [PubMed] [Google Scholar]
- 26. Aune D, Mahamat‐Saleh Y, Norat T, Riboli E. Body mass index, abdominal fatness, weight gain and the risk of urinary incontinence: a systematic review and dose‐response meta‐analysis of prospective studies. BJOG 2019; 126: 1424–33. [DOI] [PubMed] [Google Scholar]
- 27. Krhut J, Martan A, Zachoval R, Hanuš T, Švabík K, Zvara P. Impact of body mass index on treatment efficacy of mirabegron for overactive bladder in females. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016; 196: 64–8. [DOI] [PubMed] [Google Scholar]
- 28. Nozawa Y, Kato D, Tabuchi H, Kuroishi K. Safety and effectiveness of mirabegron in patients with overactive bladder in a real‐world clinical setting: a Japanese post‐marketing study. Low. Urin. Tract Symptoms 2018; 10: 122–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multiple regression analysis, by population (FAS‐UUI and FAS‐MUI analysis sets).
Data Availability Statement
Researchers may request access to anonymized participant‐level data, trial‐level data and protocols from Astellas‐sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing, see: https://clinicalstudydatarequest.com/Study‐Sponsors/Study‐Sponsors‐Astellas.aspx.


