FIGURE 3.
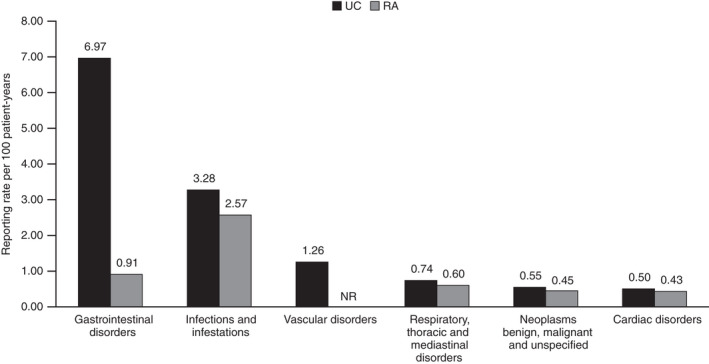
Reporting ratea of SAEs by MedDRA System Organ Class in the PMS data for tofacitinib in patients with UC and RAb. Post‐marketing exposure to tofacitinib based on estimated worldwide sales, was 8916 patient‐years for patients with UC and 34 223 patient‐years for patients with RA. MedDRA, Medical Dictionary for Regulatory Activities; NR, not reported at the time of analysis; PMS, post‐marketing surveillance; RA, rheumatoid arthritis; SAE, serious adverse event; UC, ulcerative colitis. aReporting rate is calculated by dividing the number of SAEs per MedDRA System Organ Class by the estimated patient‐years of exposure (per 100 patient‐years). bAs reported by Cohen et al. 10
