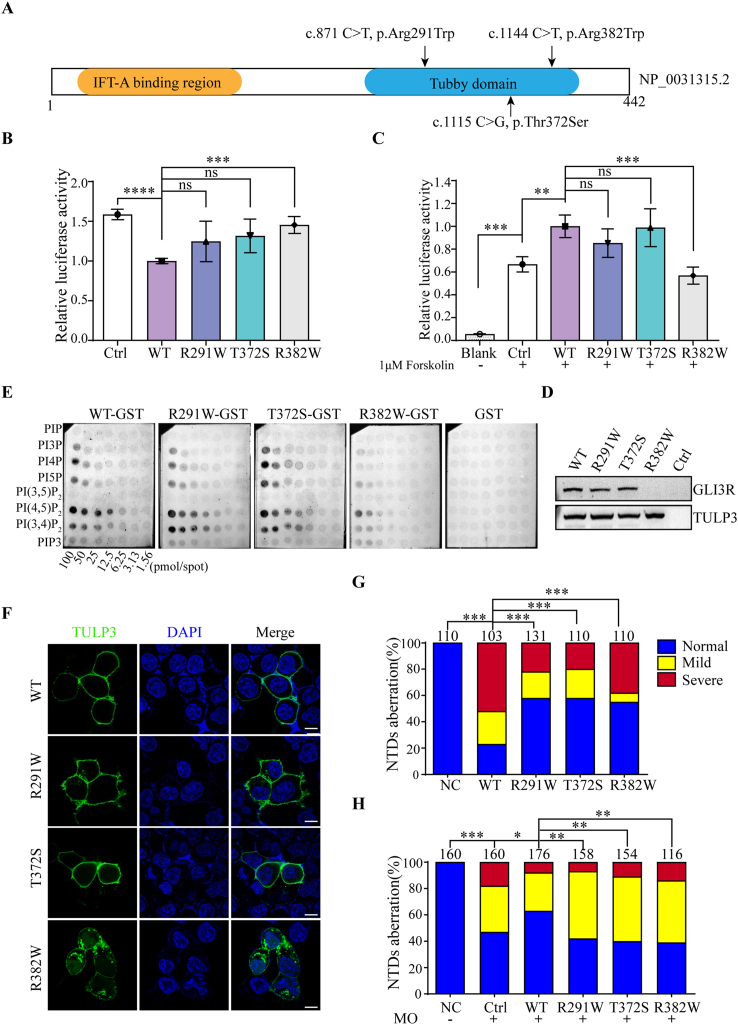
Figure 1.
Functional analysis of rare TULP3 variants identified from human neural tube defects patients. (A) Linear structure diagram of human TULP3 protein. The N-terminal IFT-A binding domain and C-terminal tubby domain are shown in orange and blue, respectively. Distributions of three variants in TULP3 protein were marked with arrows above the diagram. (B) Variant p. Arg382Trp significantly lost its suppression of Shh signaling. HEK293T cells were co-transfected with GLI-responsive luciferase reporter and empty vector (Ctrl), wild-type or mutant pCMV6-Myc-TULP3 plasmids. Twenty-four hours later, the cells were harvested and lysed for the luciferase assay. (C) Variant p. Arg382Trp significantly decreased cAMP levels. A cAMP-responsive luciferase reporter was co-transfected with empty vector (Ctrl), wild-type or mutant pCMV6-Myc-TULP3 plasmids into HEK293T cells. At 24 h post-transfection, cells were treated with 1 μM forskolin or not as indicated for another 1 h. (D) Variant p. Arg382Trp reduced the formation of GLI3R. Empty vector, wild-type or mutant pCMV6-Myc-TULP3 plasmids were transfected into HEK293T cells. Endogenous GLI3R was detected by Western blotting. All experiments were performed for three independent times, and representative results are shown as the mean ± SD. The statistical analysis used an unpaired two-tailed t-test. Asterisks above horizontal lines indicated a statistically significant difference (ns, not significant, P > 0.05, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001). (E) Variant p. Arg382Trp decreased its affinity to PtdIns(4,5)P2 and dissociated from the membrane in PIP array assay. Membranes with PtdInsPs dots in gradient concentrations were used to test the affinity of the GST-TULP3 fusion protein towards PtdInsPs. GST protein was used as a negative control. The darker dots indicate higher affinity between PtdInsPs and GST-TULP3 fusion protein. (F) Variant p. Arg382Trp resulted in subcellular localization change of TULP3. Confocal microscopy of wild-type or mutant pCMV6-EGFP-TULP3 plasmids transfected HEK293T cells at 24 h post-transfection with 63 × oil lenses. GFP (green) and DAPI (blue) represent TULP3 and nucleus, respectively. Scale bar: 10 μm (bottom white lines). (G, H) Human TULP3 variants caused severe somite development defects in zebrafish. (G) Distribution of the three categories in each group in overexpression assay. For overexpression, embryos were injected with wild-type or mutant human TULP3 mRNAs, respectively. (H) Distribution of the three categories in each group in Tulp3-MO knock down and rescue assay. To knock down endogenous Tulp3, 8 ng of Tulp3-MO was injected into each embryo. For rescue, 100 pg of wild-type or mutant human TULP3 mRNAs and 8 ng of Tulp3-MO were co-injected into each embryo. Zebrafish embryos were clustered into three categories, namely normal, mild and severe according to the severity of defects in somite shape. The distribution of the three categories in the indicated groups was calculated. The number above each column indicated the total number of embryos counted. Scale bars are 200 μm. Statistical significance (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001) was evaluated by χ2 analysis in GraphPad Prism and shown above the horizontal lines. NC, un-injected group serves as the negative control; hpf, hour post fertilization.