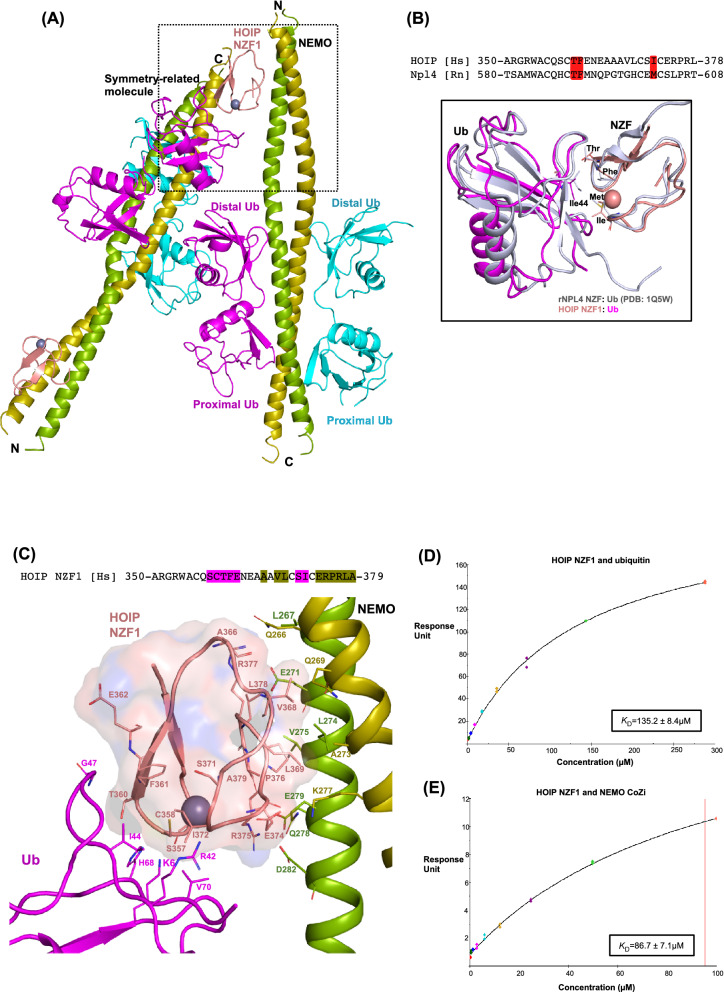
Figure 2.
HOIP NZF1 binds NEMO and ubiquitin simultaneously. (A) Crystal packing of the heteropentameric structure indicates interaction of a proximal ubiquitin from a symmetry-related molecule with HOIP NZF1. (B) Sequence alignment and superimposition of HOIP NZF1 and Npl4 NZF. The conserved residues involved in interactions with ubiquitin are highlighted in red. (C) HOIP NZF1 uses distinct surfaces for binding NEMO and ubiquitin. Amino acid residues from HOIP NZF1 interacting with NEMO or ubiquitin are highlighted green and magenta, respectively. (D,E) Surface plasmon resonance (SPR) analysis of HOIP NZF1 interaction with ubiquitin and NEMO, indicating equilibrium dissociation constant (KD) values of 135.2 ± 8.4 µM and 86.7 ± 7.1 µM (KD ± SE), respectively.