FIGURE 1.
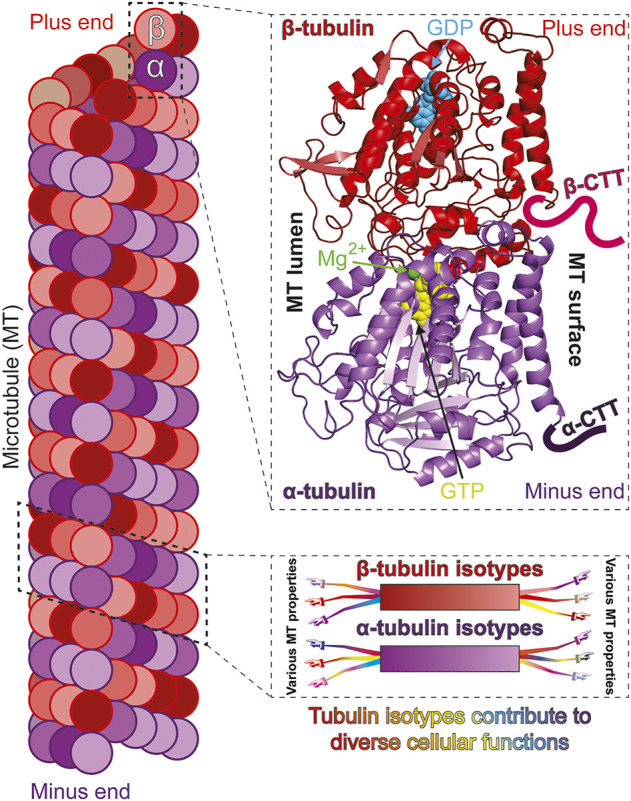
Isotypes of α- and β-tubulin construct functionally diverse microtubules. Microtubules (MTs) polymerize from “head-to-tail” binding of tubulin, a heterodimer of α- and β-tubulin subunits (left). Both α- and β-tubulin subunits bind GTP but only the β-tubulin bound GTP is hydrolysable and exchangeable [top-right; cryo-EM structure of S. cerevisiae tubulin, polymerized with GTP in vitro; PDB ID: 5W3F (Howes et al., 2017)]. The carboxy terminal tails (CTTs) are normally unstructured and are only drawn representatively and not to scale. The α- and β-tubulin subunits can be encoded by multiple genes to produce variants, or isotypes (bottom-right; color gradient represents different isotypes). Tubulin isotypes can each possess common and/or unique molecular properties which expands the ability of MTs to optimally perform diverse and specialized functions (represented by arms/hands differentially extending from isotypes).
