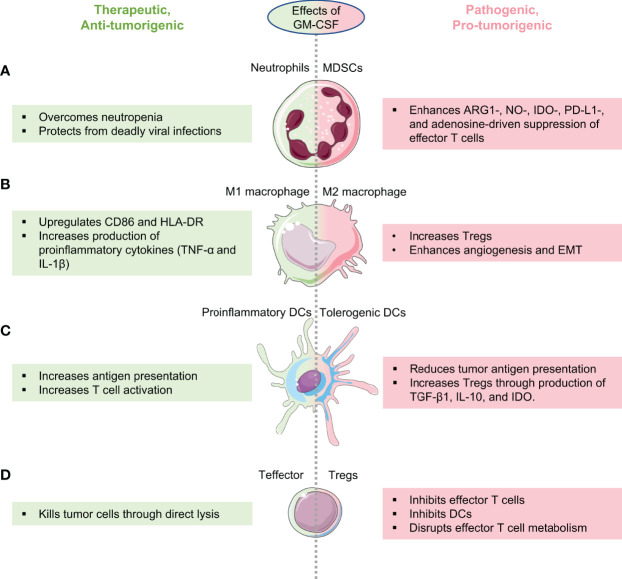
Figure 2.
Therapeutic and pathogenic effects of GM-CSF on anti-cancer immune surveillance. Schematic highlighting how GM-CSF behaves as a double-edged sword in cancer by enhancing both anti- and pro-tumorigenic immune cells depending on its expression, cancer type, and tumor immune microenvironment. GM-CSF’s role in enhancing anti-tumor immune surveillance is shown in green and its role in reprogramming immune cells to the pro-tumorigenic phenotype is shown in pink. (A) GM-CSF enhances the production of neutrophils enabling patients with cancer to flight neutropenia (left), while also having the potential to convert neutrophils to pathogenic, cancer-promoting myeloid-derived suppressor cells (MDSCs, right). (B) Depending on the cancer, GM-CSF can reprogram macrophages to the tumor suppressive M1 phenotype (left) or to the tumor-promoting M2 phenotype (right). (C) GM-CSF can convert dendritic cells in cancers to the pro-inflammatory phenotype with better antigen-presentation capabilities to cytotoxic T cells (left) or to the tolerogenic phenotype that suppress cytotoxic T cells at the expense of regulatory cells (right). (D) By regulating myeloid cells that bridge innate and adaptive immune responses as outlined in (A–C), GM-CSF can enhance the function of anti-cancer effector T cells (left) or induce regulatory T cells (right).