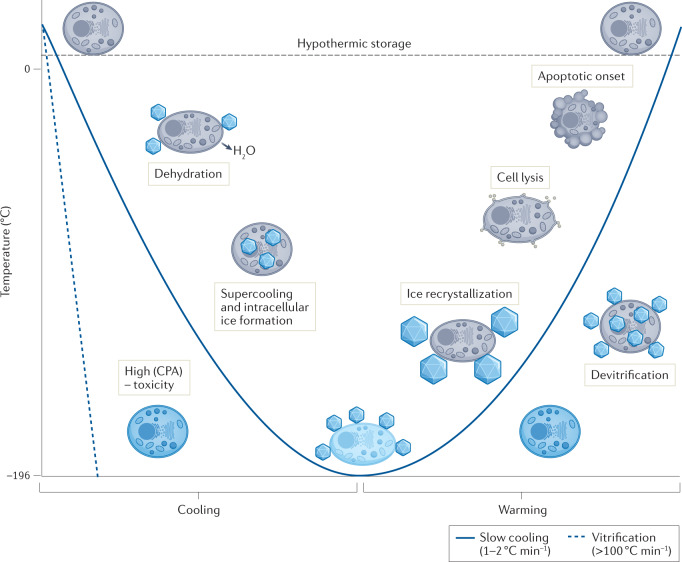
Fig. 1. Potential mechanisms of cellular damage during cryopreservation.
Several challenges occur when cells are cooled for cryogenic storage, as well during rewarming. During cooling, the formation of extracellular ice crystals leads to an osmotic imbalance across the cell membrane and subsequent cell dehydration. In the absence of extracellular ice, samples might supercool below the equilibrium freezing point, leading to an increased chance of fatal intracellular ice formation. Fast cooling rates and the addition of high concentrations of cryoprotective agents (CPA) can achieve vitrification, an amorphous, ice-free state. However, high concentrations of CPA can be toxic to cells. During warming, ice recrystallization can occur, where ice crystals grow and cause mechanical damage and cell lysis. Vitrified samples may become unstable and devitrify, leading to further ice growth. Finally, cryopreservation can induce apoptosis, leading to delayed cell death post-thaw. Note that these are all extremes, do not necessarily occur simultaneously and can be partially mitigated by the addition of cryoprotective agents. Ice crystals are not to drawn to scale for illustrative purposes.