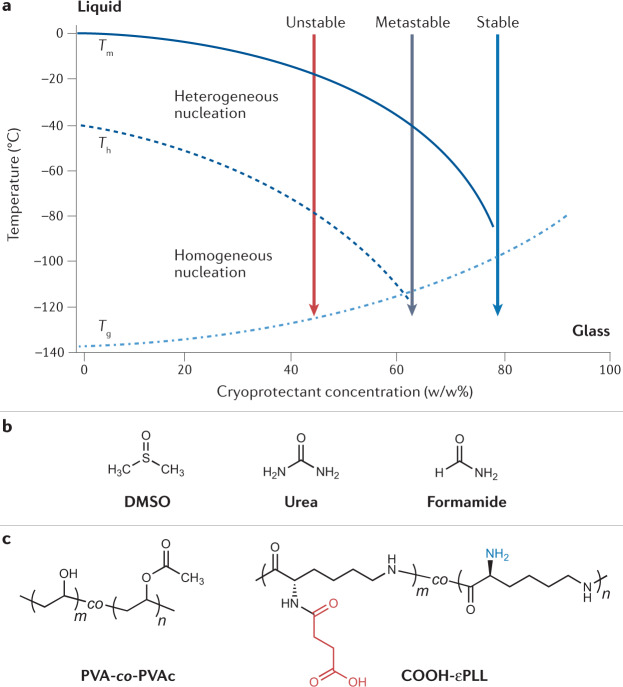
Fig. 2. Vitrification agents for cryopreservation.
To achieve vitrification, a sample must be cooled to obtain a glassy, amorphous state without heterogeneous (via foreign particles) or homogeneous (through random ordering of water molecules) ice nucleation. a | Schematic phase diagram of an aqueous sample with different concentrations of a cryoprotectant, adapted with permission from ref.43. Tm and the solid line denote the equilibrium melting temperature, Th and the dashed line denote the temperature of homogeneous ice nucleation, and Tg and the dashed/dotted line denote the glass transition temperature of the sample. For dilute samples (<40% cryoprotectant), vitrification is difficult to achieve due to the likelihood of nucleation occurring. b | Structures of small molecules that have been investigated for toxicity neutralization: dimethyl sulfoxide (DMSO), urea and formamide34. c | Macromolecular compounds such as poly(vinyl alcohol)-co-poly(vinyl acetate) (PVA-co-PVAc) copolymers and carboxylated ε-poly-L-lysine (COOH-εPLL) have been used to limit devitrification of samples during warming43. Panel a adapted with permission from ref.193, Elsevier.