Fig. 3. Ice recrystallization inhibitors.
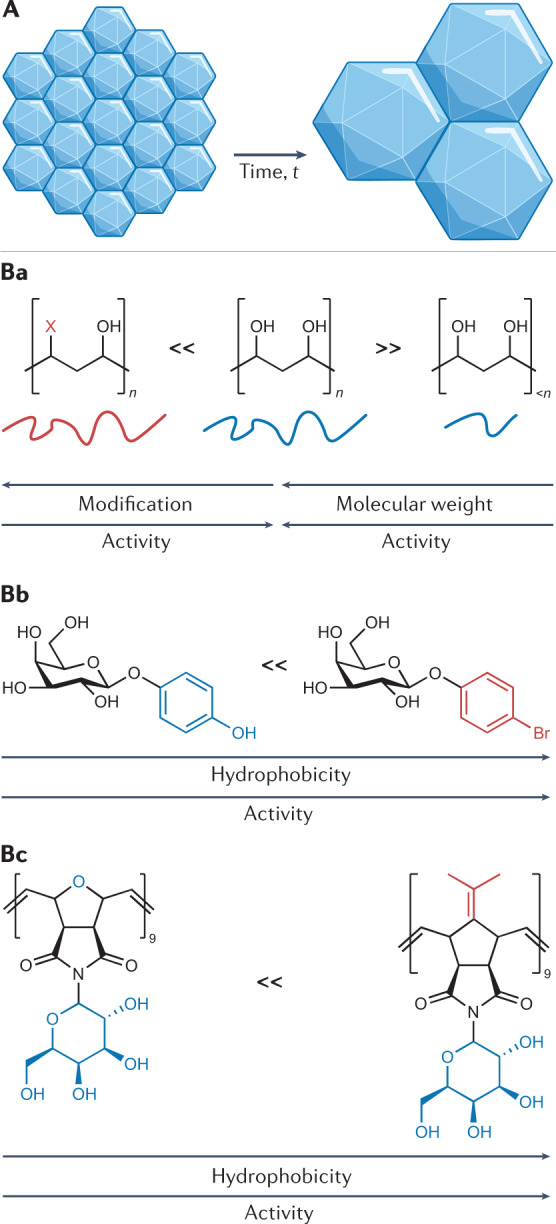
Ice recrystallization describes the growth of larger ice crystals at the expense of smaller crystals and is a form of Ostwald ripening, as depicted in A. Ice recrystallization inhibitors (IRIs) are molecules/materials that slow the growth of ice crystals, for instance, poly(vinyl alcohol) is a highly active macromolecular IRI (B). The chemical structures of IRIs are crucial to their function and the design rules can vary between classes. Removing the hydroxyl groups of PVA (for example, to add more hydrophobic groups) or decreasing the molecular weight reduces ice recrystallization inhibitory activity (Ba)69. O-aryl-glycosides (Bb) are an example of small-molecular-weight IRIs that have been shown to improve red blood cell recovery194. For this class of IRI, an increase in hydrophobicity leads to an increase in their ice recrystallization inhibitory activity. Facial amphiphilicity has been found to be crucial for a range of materials to have ice recrystallization inhibitory activity and is a feature of many IRI-proteins, but also synthetic polymers that can slow ice growth, such as those shown in Bc (ref.59).
