Figure 1.
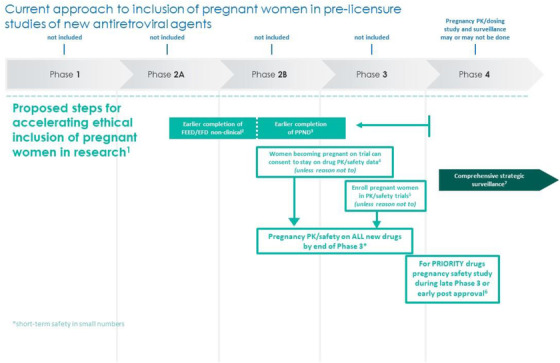
Framework and approaches to expedite the timeline for the study of new antiretrovirals drugs in pregnancy. (1) Involve women of childbearing potential living with HIV from the identification of research questions through the study design, recruitment, conduct and dissemination of results. (2) Perform non‐clinical developmental and reproductive toxicology studies (DART) earlier during drug development for all new HIV agents. Fertility and early embryonic development (FEED) and embryo‐foetal development (EFD) studies should be completed during or no later than the end of the phase 2 registrational trials. (3) Pre‐ and postnatal development (PPND) studies should be completed during early phase 3 or no later than the end of phase 3 registrational trial. (4) Women who become pregnant in registrational trials should be given the option to make an informed choice to stay on study drug once early non‐clinical FEED and EFD studies are completed, with no negative signals and dosing is established in non‐pregnant people. (5) Enrol pregnant women in specific studies to determine pharmacokinetic (PK) and preliminary safety as soon as late non‐clinical PPND studies are completed with no negative signals for all new HIV agents. (6) Investigate adverse maternal, pregnancy and birth outcomes through dedicated pregnancy safety studies for all new priority HIV agents identified through CADO as soon as dosing is confirmed. (7) Expand systematic and rigorous active safety surveillance studies to enable systematic and rapid detection of adverse birth outcomes and rare events, such as birth defects associated with exposure to antiretrovirals during pregnancy.
