Figure 4.
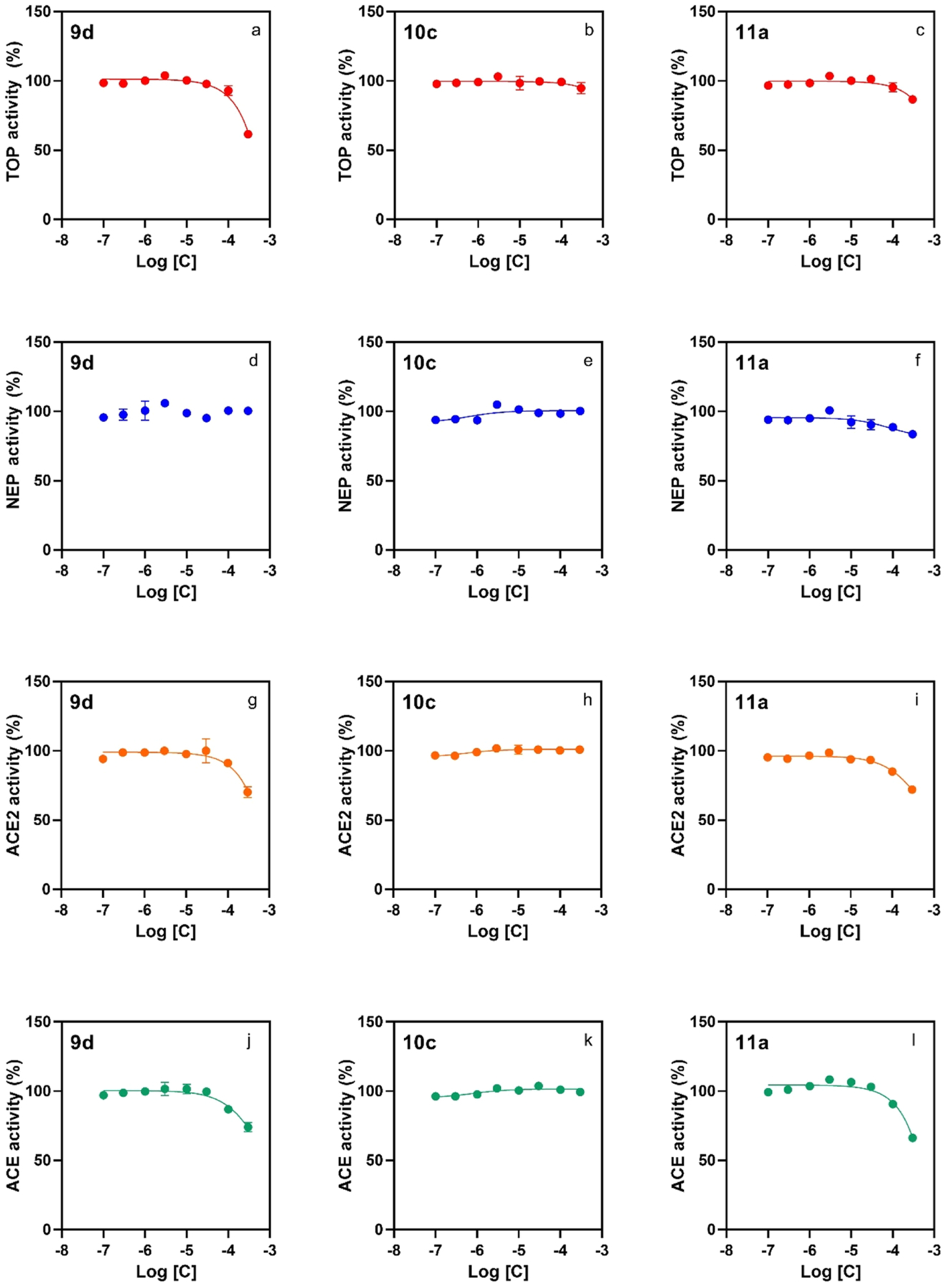
Peptidomimetic compounds 9d, 10c, and 11a have a low effect on the catalytic activity of human recombinant peptidases, illustrating selectivity toward activation of Nln over the related peptidases TOP, NEP, ACE2, and ACE. All panels document concentration-dependent effect of the indicated compounds on hydrolysis of a respective quenched fluorescent substrate (n = 4, mean ± SD): Mca-Pro-Leu-Gly-Pro-D-Lys(DNP)-OH at 15 μM for TOP (panels a–c), Mca-Arg-Pro-Pro-Gly-Phe-Ser-Ala-Phe-Lys(Dnp)-OH at 10 μM for NEP (panels d–f), Mca-Ala-Pro-Lys-(Dnp)-OH at 10 μM for ACE2 (panels g–i), and angiotensin-converting enzyme (ACE; panels j–l). In all panels, the initial velocity of the hydrolysis in the absence of either compound corresponds to 100% on the vertical axis.
