Figure 5.
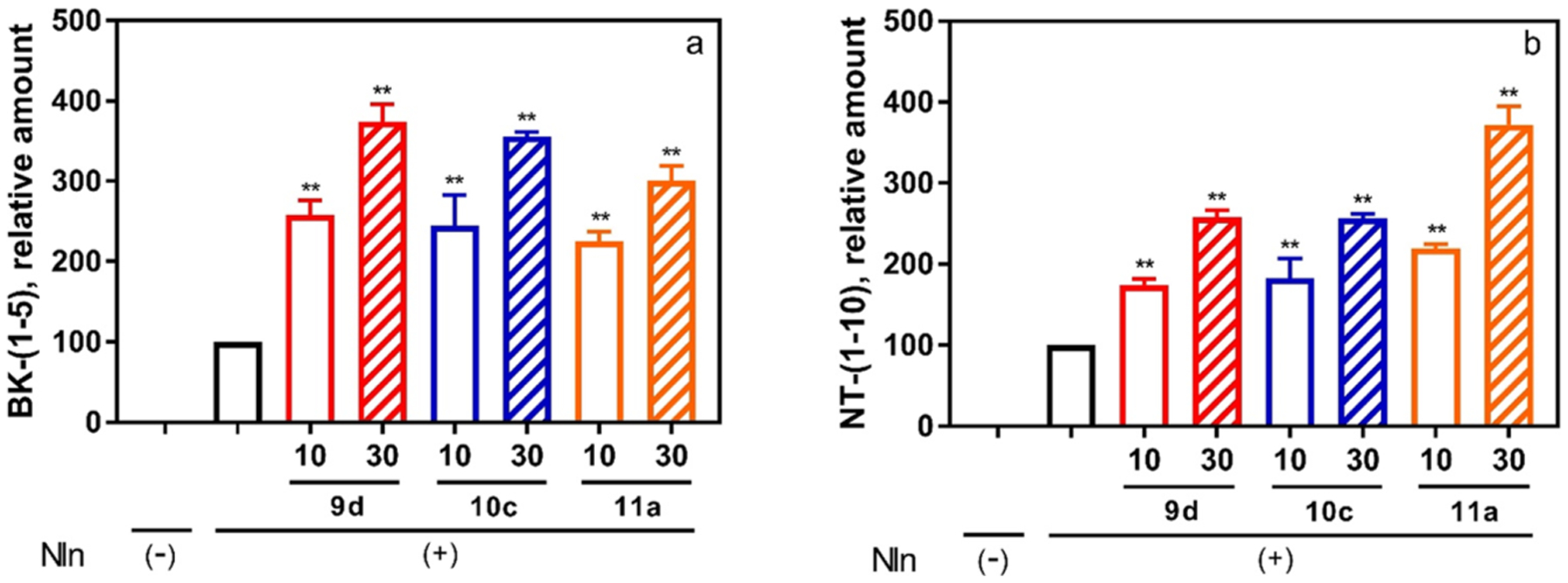
Effect of peptidomimetic compounds 9d, 10c, and 11a on the hydrolysis of Nln substrates BK and NT illustrates that activation of Nln is not an artifact of the synthetic substrate and translates to the natural system. Rat recombinant neurolysin was incubated with one of the endogenous peptides (20 μM) in the absence or presence of the test compounds (10 or 30 μM). The formation of (A) bradykinin-(1–5) (BK-(1–5)) and (B) neurotensin-(1–10) (NT-(1–10)) was documented by mass spectrometric analysis (n = 3, mean ± SD are presented; **, p < 0.001 compared to Nln-alone condition). In both panels, Nln(−) corresponds to a condition where the respective peptide substrate was incubated in assay buffer alone (i.e., no Nln, hence no product formation). Likewise, Nln(+) corresponds to a condition where the recombinant peptidase was present in the assay buffer.
