Figure 4.
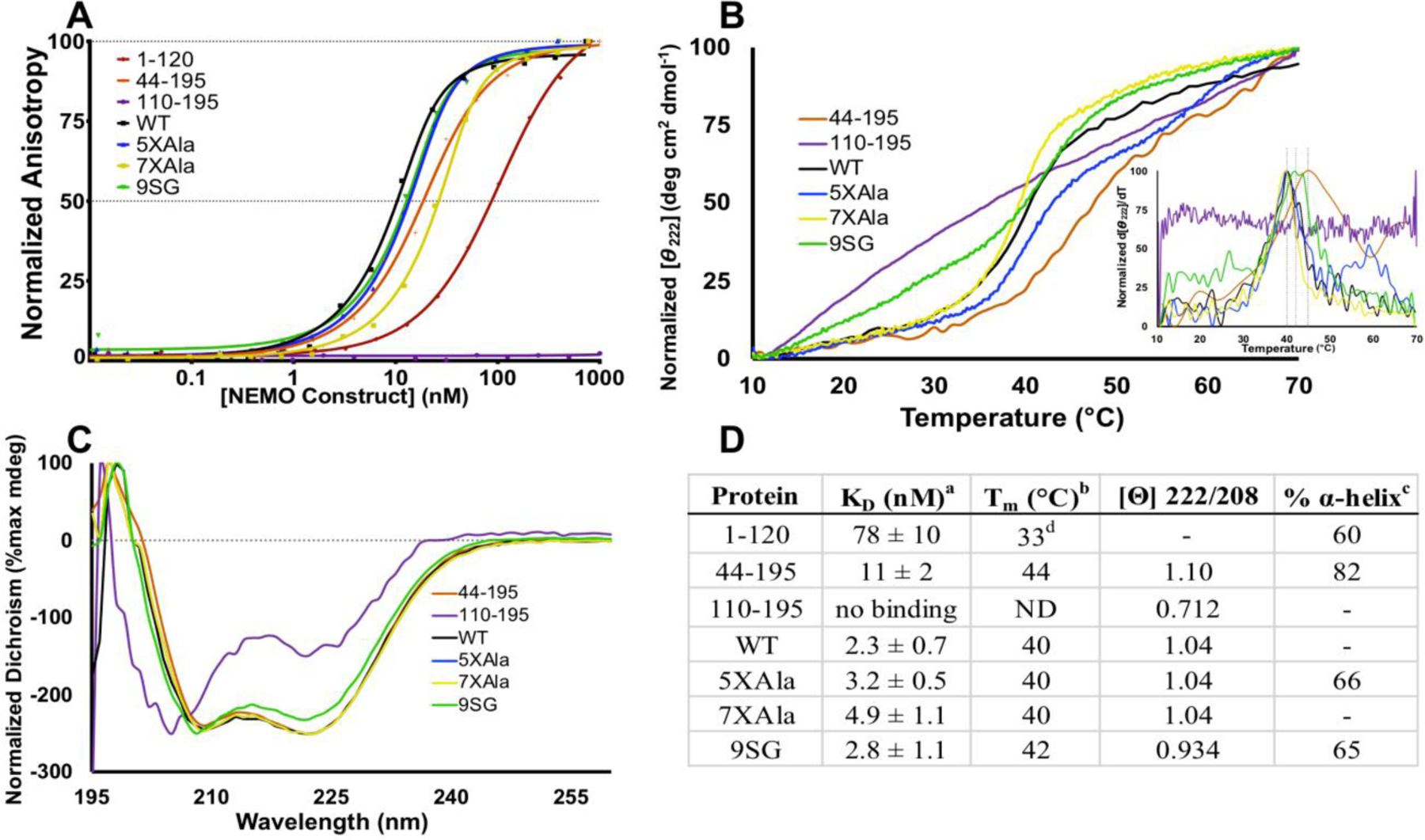
The IVD contributes to NEMO-IKKβ binding affinity and NEMO thermal stability. (A) Fluorescence anisotropy binding assays. The indicated NEMO proteins were titrated from 0.01 nM – 1000 nM, with FITC-labeled IKKβ(701–745) kept constant at 15 nM. Results are representative of three independent experiments performed with triplicate samples. (B) The indicated NEMO proteins were subjected to thermal denaturation, as monitored by CD. An increase in signal at Θ = 222 nm corresponds to loss of secondary structure. Inset: first derivative of melting curve used to identify Tm value. (C) CD spectra of NEMO constructs determined at 10 °C. The negative peak with minima at 208 and 222 nm is indicative of α-helical content. (D) Table of key results. aKD for binding FITC-IKKβ(701–745) measured using the FA binding assay. bDetermined by CD, monitoring change in Θ222 as temperature was increased at 1 °C/min. c The % α-helical content was determined from the CD spectrum in aqueous buffer compared with that in 90% TFE.d The Tm for NEMO(1–120) was reported previously, and is shown here for reference28.
