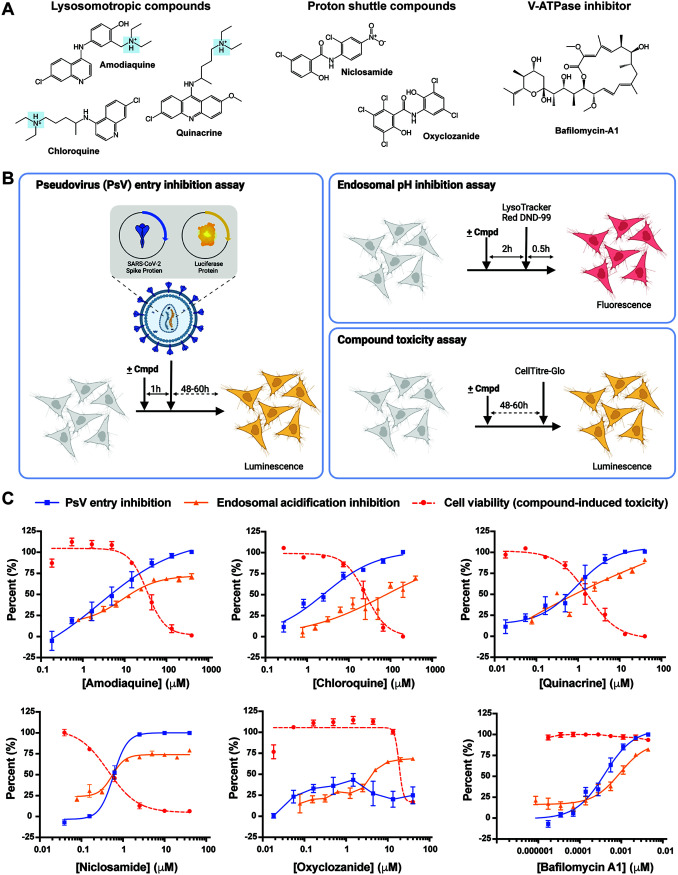
FIG 1.
Identification of bafilomycin A1 as a safe and potent inhibitor of SARS-CoV-2 pseudovirus (PsV) entry into mammalian cells. (A) Chemical structure of three groups of endosomal acidification modifiers: lysosomotropic compounds (amodiaquine, chloroquine, and quinacrine), proton shuttles (niclosamide and oxyclozanide), and a specific V-ATPase inhibitor (bafilomycin A1). The highlighted tertiary ammonium cation of the lysosomotropic compounds is a characteristic structure of phospholipidosis-causing compounds. (B) Assays testing pseudoviral entry inhibition (left), endosomal acidification inhibition (top-right), and compound-mediated cell cytotoxicity (bottom-right) of each compound. (C) Comparison of inhibition of PsV entry, inhibition of endosomal acidification, and cell viability by each tested compound against HeLa-ACE2 cells. All experiments were conducted in triplicate, at the minimum, (n = 3 to 14).