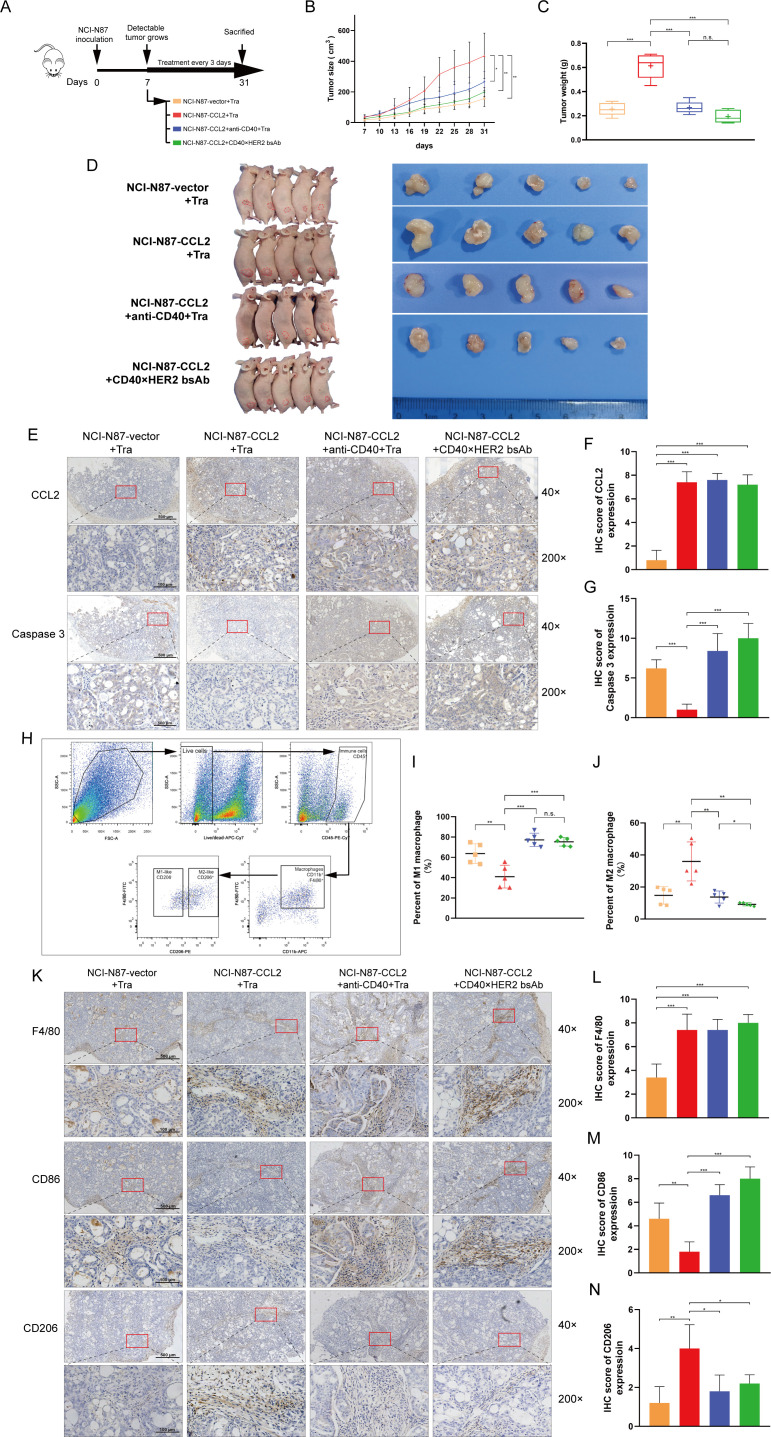
Figure 5.
CD40 ×HER2 bsAb overcame trastuzumab resistance in the xenograft model. (A) Experimental scheme of CD40 ×HER2 bsAb treatment in NCI-N87-tumor-bearing Balb/c nude mice. The different treatment groups included: (1) the stable control NCI-N87 cells with the trastuzumab treatment (NCI-N87-vector+Tra group), (2) the stable CCL2 overexpressing NCI-N87 cells with the trastuzumab treatment (NCI-N87-CCL2+Tra group), (3) the stable CCL2 overexpressing NCI-N87 cells with the trastuzumab and agonistic anti-CD40 mAb treatment (NCI-N87-CCL2+anti-CD40+Tra group), (4) the stable CCL2 overexpressing NCI-N87 cells with CD40 ×HER2 bsAb treatment (NCI-N87-CCL2+CD40×HER2 bsAb group). (B–D) Tumor growth curves (B), tumor weight (C) and tumor volume images (D) of each group were shown. (E–G) IHC staining for CCL2 and caspase three was presented (E), and the IHC scores for CCL2 (F) and caspase 3 (G) were statistically analyzed. (H) The gating strategy to identify CD45+ CD11b+ F4/80+ CD206+/- TAMs in mouse tumor tissues. (I, J) The infiltrating CD45+ CD11b+ F4/80+ CD206- (I) or CD45+ CD11b+ F4/80+ CD206+ (J) TAMs percentages in the whole cell counts detected by flow cytometry were statistically analyzed. (K–N) IHC staining for F4/80, CD86, and CD206 was presented to show the infiltrating TAMs in mouse tumors (K). IHC scores for F4/80 (L), CD86(M) and CD206 (N) were statistically analyzed. n=5 per group. tra, trastuzumab treatment. Scale bar, ×40, 500 µm; ×200, 100 µm. *P<0.05, **p<0.01, ***p<0.001. bsAb, bispecific antibody; IHC, immunohistochemistry; ns, not significant.