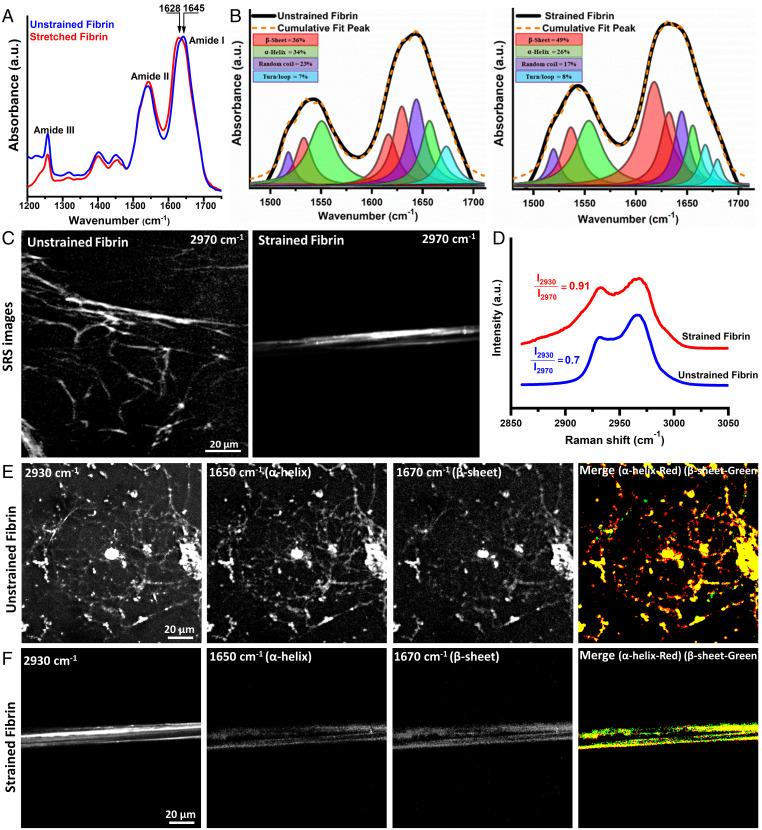
Fig. 2.
Tensile strain causes fibrin molecular and structural unfolding. (A) ATR-IR spectra of unstrained and 100% strained fibrin showing amide I to III bands with a clear amide I peak shift showing structural changes. (B) Decomposition of amide I and II modes from spectra in (A) into constituent peaks corresponding to different protein secondary structures shows the increased β-sheet content with strain. (C) SRS CH stretch (2,970 cm−1) image, (D) CH stretch spectra from 2,850 to 3,050 cm−1 of unstrained and strained fibrin. (E) and (F) SRS images from strained and unstrained fibrin acquired at the CH3 mode (2,930 cm−1), α-helix mode (1,650 cm−1), β-sheet mode (1,670 cm−1), and thresholded overlapped α-helix (red) and β-sheet (green) images. Imaging conditions were identical for all SRS images at the respective wavenumbers.