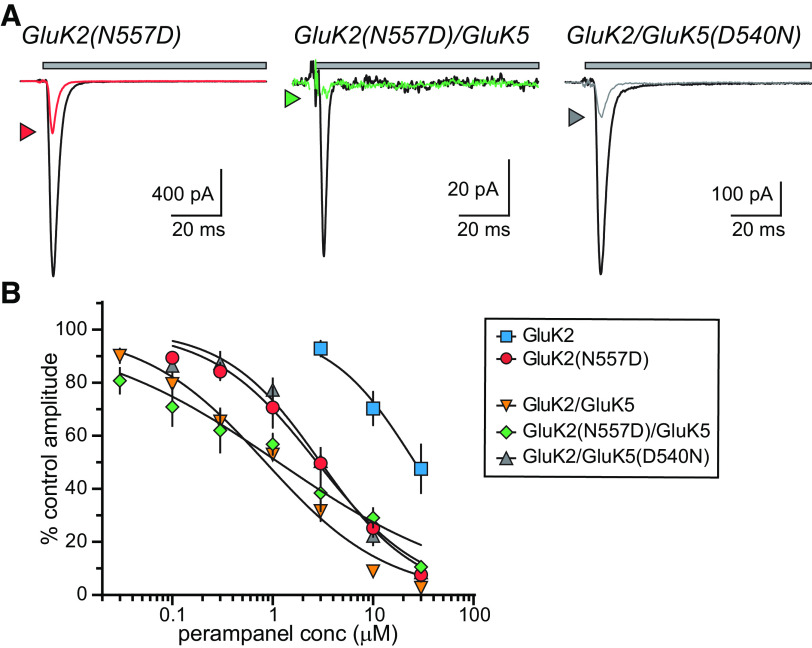
Figure 4.
The role of a key amino acid in PMP inhibition of KARs. A, Representative currents from GluK2 and GluK2/GluK5 KARs containing mutations that swap the respective amino acids at a key binding site determinant (N557 in GluK2, D540 in GluK5). Currents were evoked by glutamate (10 mm for 100 ms) either alone (black lines) or in the presence of PMP (10 µm, colored lines) for 100 ms. Top, The gray bar denotes glutamate application. B, Normalized mean amplitudes of currents evoked from GluK2(N557D), GluK2(N557D)/GluK5, and GluK2/GluK5(D540N) at a range of PMP concentrations were best fit with logistic curves with variable IC50 and Hill slopes. The data and fitted curves for GluK2 and GluK2/GluK5 KARs are identical to those in Figure 2 and are shown for the sake of comparison. IC50 values, 95% confidence intervals, and statistical analyses are shown in Table 1. Reciprocal swaps at this site increased sensitivity of homomeric GluK2 KARs to PMP attenuated but did not eliminate PMP inhibition of GluK5(D540N)-containing KARs.