Table 1.
Characterization of PARP inhibitors with preclinical data in hematological malignancies
| Name of Inhibitor | Structure | IC50 | In vitro effects | In vivo effects | Stage of Development |
|---|---|---|---|---|---|
| Talazoparib (BMN673) |
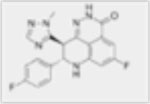
|
0.6nM | Combination with DNMTis synergistic toxicity and reduced colony formation. | Combination with DNMTis resulted in increase in overall survival and significant anti-tumor response. | Recruiting/Phase 1 |
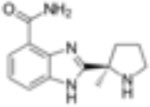
| Olaparib (Ku-0059436) |
|
5nM | Anti-proliferative and apoptotic events in AML cell lines, accumulation of S phase cell cycle arrest. Synergistic anti-leukemic effects in combination with HDACi, WEE1 inhibition, GO. | Increase in overall survival in combination with AZD1775 | Phase 1 Phase 2 |
| PJ34 HCl |
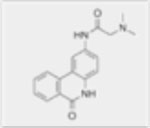
|
N/A | Dose-dependent decrease in cell viability, induces apoptosis in AML cell lines. | Alleviated hepatomegaly and splenomegaly and reduced level of C1498 cells. | N/A |
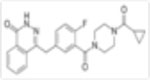
| Veliparib (ABT 888) |
|
5nM | Anti-leukemic activity in combination with DNMTis. Synergistic anti-proliferative effects in combination with TRAIL. Enhances activity of TMZ. | Veliparib combined with Cisplatin achieved significant tumor growth inhibition. | Phase I Active not yet recruiting |
| Niraparib (MK-4827) |
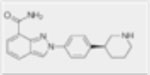
|
4nM | N/A | N/A | N/A |
| Rucaparib (AG-0146991,PF-01367338) |
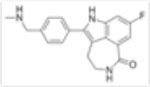
|
1nM | Increased sensitivity in AML cell lines at 0.1–100M. | Significant improvement in overall survival in combination with fluororacil. | N/A |
