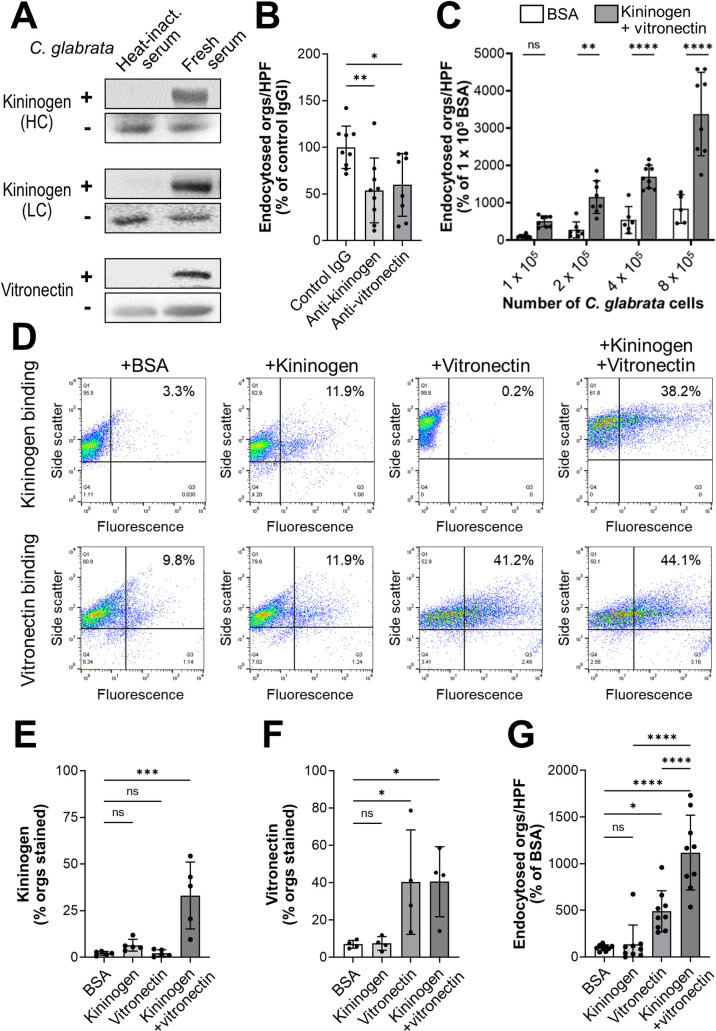
Fig 5. High molecular weight kininogen and vitronectin function as bridging molecules.
(A) Western blots showing that the heavy chain (HC) and light chain (LC) of high molecular weight kininogen and vitronectin bind to C. glabrata cells that have been incubated in fresh human serum. In each pair of blots, the upper panel shows the proteins that were eluted from C. glabrata and lower panel shows the proteins present in serum in the absence of C. glabrata. (B) Effects of anti-kininogen and anti-vitronectin antibodies on the endocytosis of serum-coated C. glabrata by human endothelial cells. (C) Endocytosis of the indicated number of C. glabrata cells that had been coated with either BSA or kininogen and vitronectin. (D) Flow cytometric detection of the binding of kininogen (top row) and vitronectin (bottom row) to C. glabrata cells that had been incubated for 1 h with BSA without kininogen or vitronectin, kininogen alone, vitronectin alone, or kininogen and vitronectin. Numbers in the upper right hand corner indicate the percentage of positive cells. Results are representative of 5 (kininogen) or 4 (vitronectin) separate experiments, each of which analyzed 10,000 cells. (E-F) Summary of combined flow cytometry results showing the binding of kininogen (E) and vitronectin (F) to C. glabrata cells. (G) Endocytosis of C. glabrata cells that had been coated with the indicated proteins. Data in (B), (C), and (G) are the mean ± SD of 3 experiments each performed in triplicate. Orgs/HPF, organisms per high power field; ns, not significant; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by ANOVA with the Dunnett’s test for multiple comparisons.